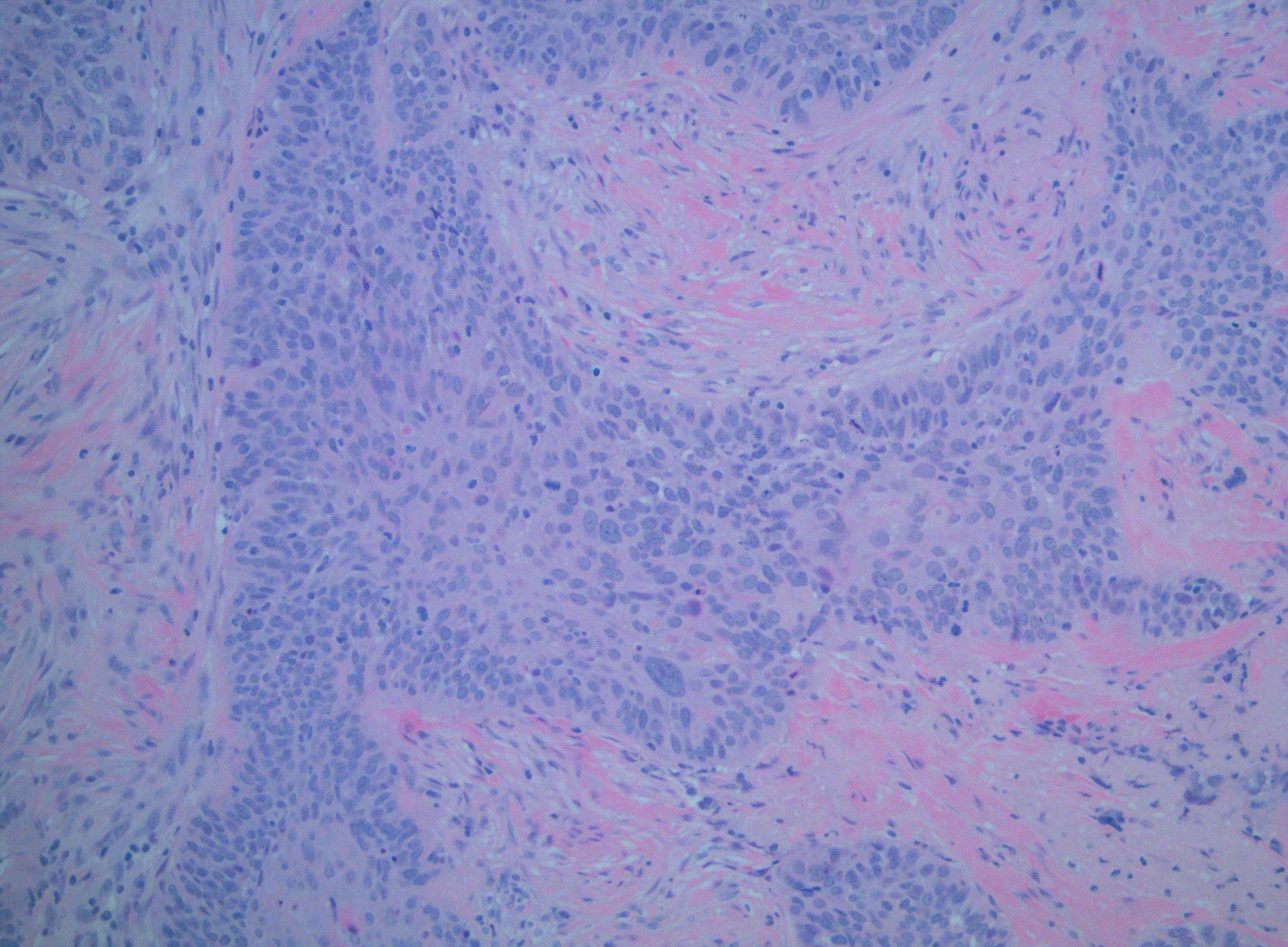
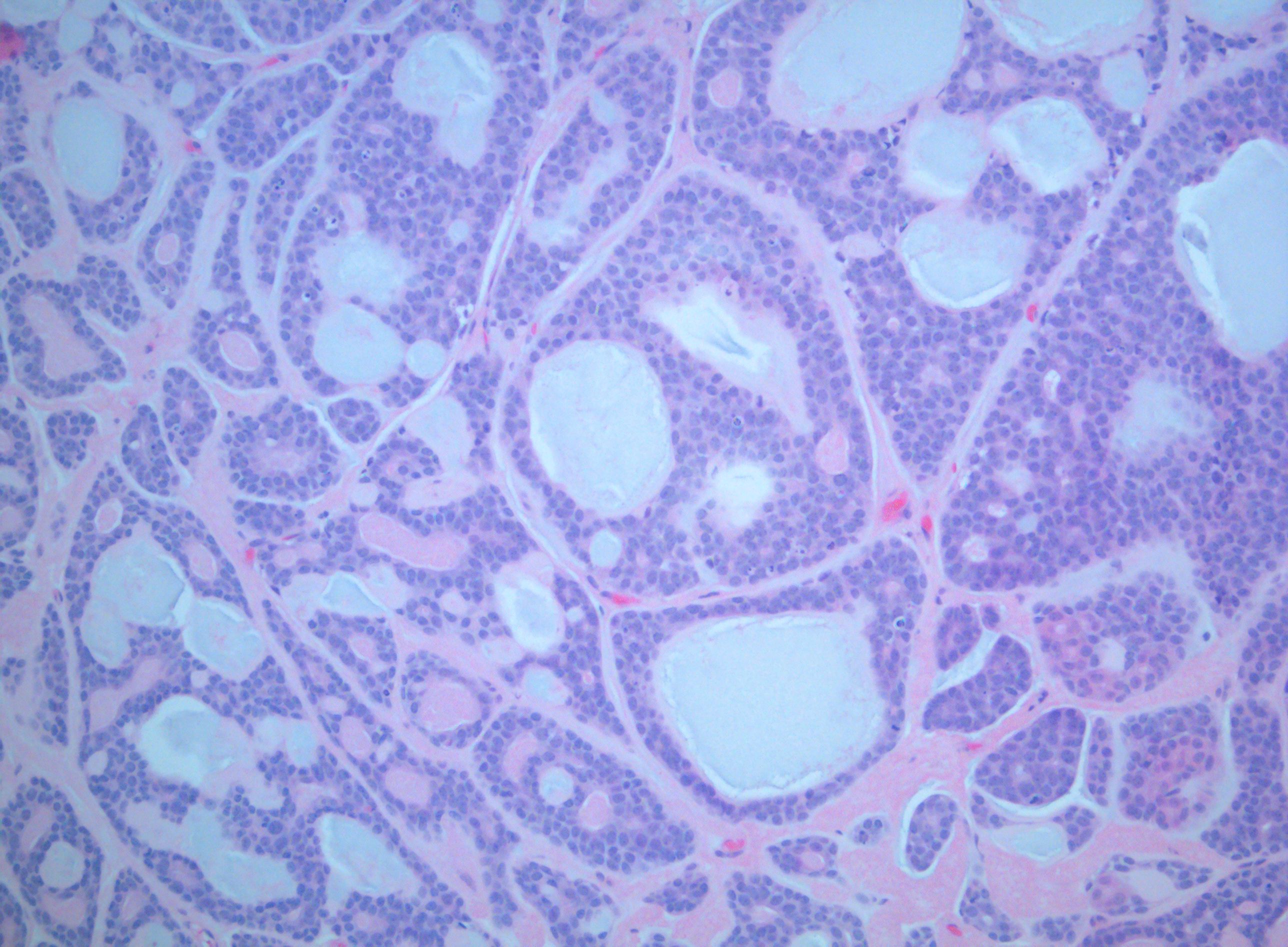
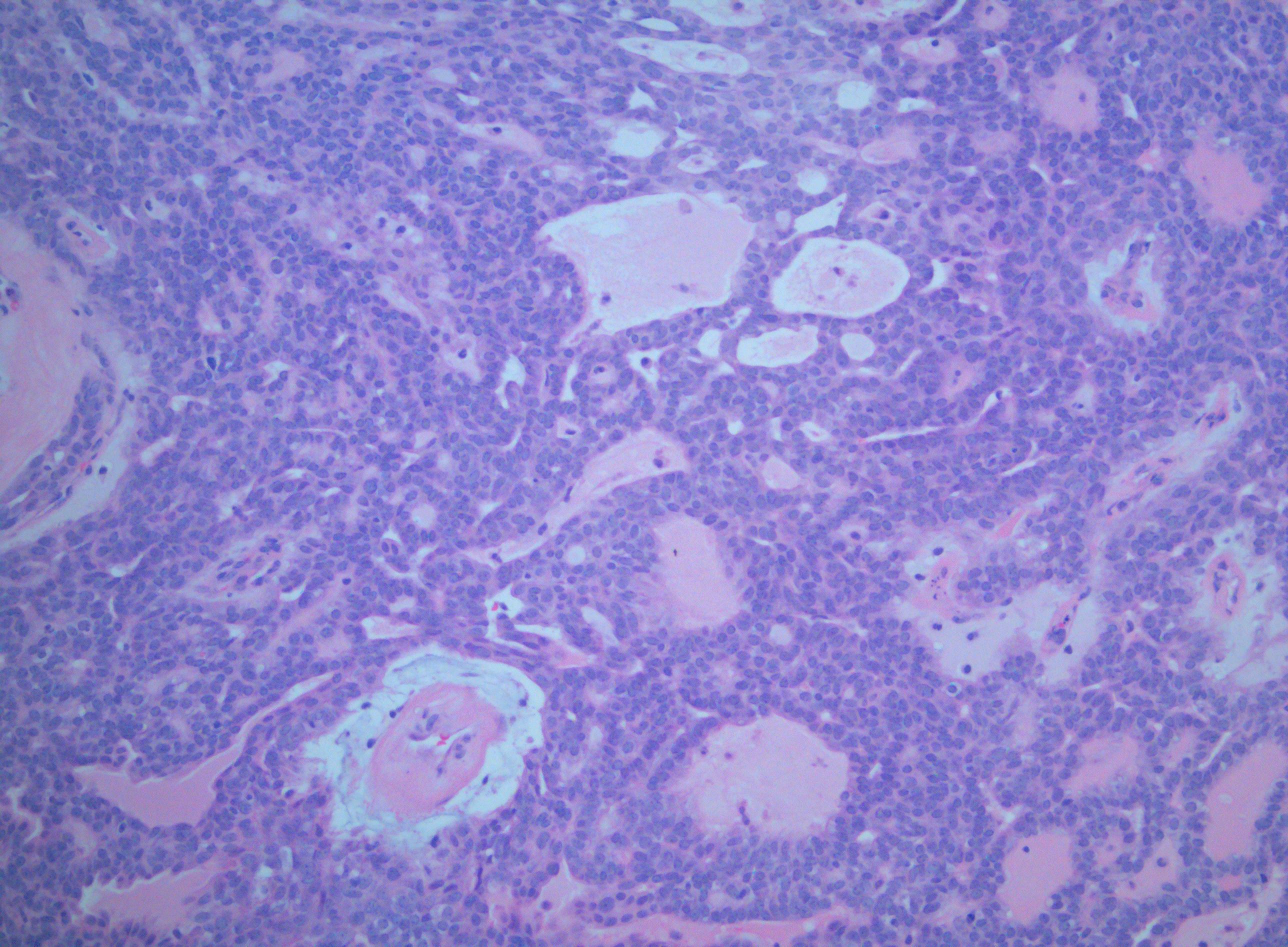
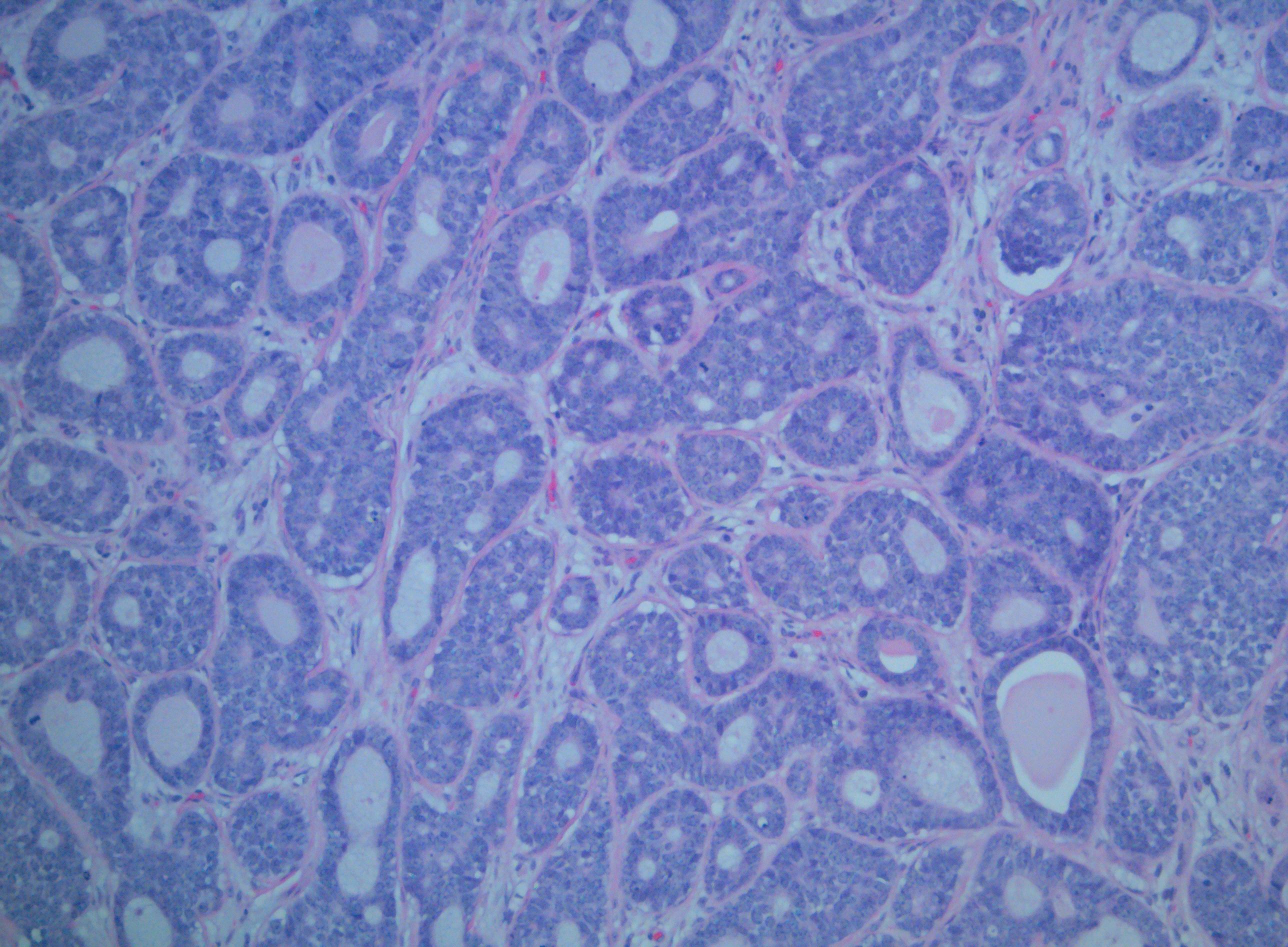
[1]
Aydil U, Kızıl Y, Bakkal FK, Köybaşıoğlu A, Uslu S. Neoplasms of the hard palate. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2014 Mar:72(3):619-26. doi: 10.1016/j.joms.2013.08.019. Epub 2013 Oct 16
[PubMed PMID: 24139293]
[2]
Kato H, Kanematsu M, Makita H, Kato K, Hatakeyama D, Shibata T, Mizuta K, Aoki M. CT and MR imaging findings of palatal tumors. European journal of radiology. 2014 Mar:83(3):e137-46. doi: 10.1016/j.ejrad.2013.11.028. Epub 2013 Dec 12
[PubMed PMID: 24377674]
[3]
Kim CJ, Freedman DM, Curtis RE, Berrington de Gonzalez A, Morton LM. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leukemia & lymphoma. 2013 Aug:54(8):1691-7. doi: 10.3109/10428194.2012.753543. Epub 2012 Dec 31
[PubMed PMID: 23193978]
[4]
Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, Tedesco B. Epidemiology of mouth cancer in 1989: a review. Journal of the Royal Society of Medicine. 1990 Nov:83(11):724-30
[PubMed PMID: 2250273]
[5]
Sanghvi LD, Rao DN, Joshi S. Epidemiology of head and neck cancers. Seminars in surgical oncology. 1989:5(5):305-9
[PubMed PMID: 2814139]
[6]
Pindborg JJ, Mehta FS, Gupta PC, Daftary DK, Smith CJ. Reverse smoking in Andhra Pradesh, India: a study of palatal lesions among 10,169 villagers. British journal of cancer. 1971 Mar:25(1):10-20
[PubMed PMID: 5581290]
[7]
van der Eb MM, Leyten EM, Gavarasana S, Vandenbroucke JP, Kahn PM, Cleton FJ. Reverse smoking as a risk factor for palatal cancer: a cross-sectional study in rural Andhra Pradesh, India. International journal of cancer. 1993 Jul 9:54(5):754-8
[PubMed PMID: 8325705]
Level 2 (mid-level) evidence
[8]
QUIGLEY LF Jr,COBB CM,HUNT EE Jr, MEASUREMENT OF ORAL AND BURNING ZONE TEMPERATURES DURING CONVENTIONAL AND REVERSE CIGARETTE SMOKING. Archives of oral biology. 1965 Jan-Feb;
[PubMed PMID: 14262158]
[9]
Marshall J, Graham S, Mettlin C, Shedd D, Swanson M. Diet in the epidemiology of oral cancer. Nutrition and cancer. 1982:3(3):145-9
[PubMed PMID: 7134009]
[10]
Singhvi HR, Malik A, Chaturvedi P. The Role of Chronic Mucosal Trauma in Oral Cancer: A Review of Literature. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2017 Jan-Mar:38(1):44-50. doi: 10.4103/0971-5851.203510. Epub
[PubMed PMID: 28469336]
[11]
Ustrell-Borràs M, Traboulsi-Garet B, Gay-Escoda C. Alcohol-based mouthwash as a risk factor of oral cancer: A systematic review. Medicina oral, patologia oral y cirugia bucal. 2020 Jan 1:25(1):e1-e12. doi: 10.4317/medoral.23085. Epub 2020 Jan 1
[PubMed PMID: 31655832]
Level 1 (high-level) evidence
[12]
Mathur R, Singhavi HR, Malik A, Nair S, Chaturvedi P. Role of Poor Oral Hygiene in Causation of Oral Cancer-a Review of Literature. Indian journal of surgical oncology. 2019 Mar:10(1):184-195. doi: 10.1007/s13193-018-0836-5. Epub 2018 Dec 7
[PubMed PMID: 30948897]
[13]
Lin HH, Limesand KH, Ann DK. Current State of Knowledge on Salivary Gland Cancers. Critical reviews in oncogenesis. 2018:23(3-4):139-151. doi: 10.1615/CritRevOncog.2018027598. Epub
[PubMed PMID: 30311570]
[14]
Luo SD, Su CY, Chuang HC, Huang CC, Chen CM, Chien CY. Estrogen receptor overexpression in malignant minor salivary gland tumors of the sinonasal tract. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009 Jul:141(1):108-13. doi: 10.1016/j.otohns.2009.03.003. Epub 2009 May 5
[PubMed PMID: 19559968]
[15]
Ram H, Mohammad S, Husain N, Devi S, Gupta PN. Metastatic malignant melanoma of palate: A review of literature and report of an unusual case. National journal of maxillofacial surgery. 2010 Jan:1(1):63-6. doi: 10.4103/0975-5950.69165. Epub
[PubMed PMID: 22442554]
Level 3 (low-level) evidence
[16]
Vikey A,Kapoor P,Kathariya R,Vikey D,Kukreja I, Malignant melanoma of gingiva: Report of a rare case. Indian journal of dentistry. 2015 Jul-Sep;
[PubMed PMID: 26392736]
Level 3 (low-level) evidence
[17]
Tacastacas JD, Bray J, Cohen YK, Arbesman J, Kim J, Koon HB, Honda K, Cooper KD, Gerstenblith MR. Update on primary mucosal melanoma. Journal of the American Academy of Dermatology. 2014 Aug:71(2):366-75. doi: 10.1016/j.jaad.2014.03.031. Epub 2014 May 6
[PubMed PMID: 24815565]
[18]
Tan WC, Chan LC. Kaposi's sarcoma: case report and treatment options. The Medical journal of Malaysia. 2011 Oct:66(4):383-4
[PubMed PMID: 22299569]
Level 3 (low-level) evidence
[19]
Searles GE, Markman S, Yazdi HM. Primary oral Kaposi's sarcoma of the hard palate. Journal of the American Academy of Dermatology. 1990 Sep:23(3 Pt 1):518-9
[PubMed PMID: 2212159]
[21]
Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, Hartge P, Morton LM. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992-2009. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Jun:22(6):1069-78. doi: 10.1158/1055-9965.EPI-13-0040. Epub 2013 Apr 17
[PubMed PMID: 23595542]
[22]
Filipovich AH, Mathur A, Kamat D, Shapiro RS. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer research. 1992 Oct 1:52(19 Suppl):5465s-5467s
[PubMed PMID: 1327508]
[23]
Ekström-Smedby K. Epidemiology and etiology of non-Hodgkin lymphoma--a review. Acta oncologica (Stockholm, Sweden). 2006:45(3):258-71
[PubMed PMID: 16644568]
[24]
Cleghorn FR, Manns A, Falk R, Hartge P, Hanchard B, Jack N, Williams E, Jaffe E, White F, Bartholomew C. Effect of human T-lymphotropic virus type I infection on non-Hodgkin's lymphoma incidence. Journal of the National Cancer Institute. 1995 Jul 5:87(13):1009-14
[PubMed PMID: 7629870]
[25]
Knowles DM, Cesarman E. The Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) in Kaposi's sarcoma, malignant lymphoma, and other diseases. Annals of oncology : official journal of the European Society for Medical Oncology. 1997:8 Suppl 2():123-9
[PubMed PMID: 9209655]
[26]
Engels EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, Whitby D, Colt JS, Hartge P. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. International journal of cancer. 2004 Aug 10:111(1):76-80
[PubMed PMID: 15185346]
Level 2 (mid-level) evidence
[27]
Chiu BC, Cerhan JR, Folsom AR, Sellers TA, Kushi LH, Wallace RB, Zheng W, Potter JD. Diet and risk of non-Hodgkin lymphoma in older women. JAMA. 1996 May 1:275(17):1315-21
[PubMed PMID: 8614116]
[28]
de Sanjosé S, Benavente Y, Nieters A, Foretova L, Maynadié M, Cocco PL, Staines A, Vornanen M, Boffetta P, Becker N, Alvaro T, Brennan P. Association between personal use of hair dyes and lymphoid neoplasms in Europe. American journal of epidemiology. 2006 Jul 1:164(1):47-55
[PubMed PMID: 16731576]
[29]
Tabrizi R, Aliabadi E, Maleki MJ, Barouj MD. Association between clinical features, treatment, and recurrence rate of adenoid cystic carcinoma of the palate: a 10-year retrospective study. The British journal of oral & maxillofacial surgery. 2016 Jul:54(6):648-51. doi: 10.1016/j.bjoms.2016.03.023. Epub 2016 Apr 16
[PubMed PMID: 27094499]
Level 2 (mid-level) evidence
[30]
Ellington TD, Henley SJ, Senkomago V, O'Neil ME, Wilson RJ, Singh S, Thomas CC, Wu M, Richardson LC. Trends in Incidence of Cancers of the Oral Cavity and Pharynx - United States 2007-2016. MMWR. Morbidity and mortality weekly report. 2020 Apr 17:69(15):433-438. doi: 10.15585/mmwr.mm6915a1. Epub 2020 Apr 17
[PubMed PMID: 32298244]
[31]
Arya S, Rane P, Deshmukh A. Oral cavity squamous cell carcinoma: role of pretreatment imaging and its influence on management. Clinical radiology. 2014 Sep:69(9):916-30. doi: 10.1016/j.crad.2014.04.013. Epub 2014 Jun 5
[PubMed PMID: 24908285]
[32]
Sugimura M, Sakamoto T, Tsubakimoto M, Mori M, Kawakatsu K. Analysis of 102 lesions diagnosed as "palatal tumor" in a spot diagnosis. International journal of oral surgery. 1975 Sep:4(4):143-50
[PubMed PMID: 809374]
[33]
Givi B, Eskander A, Awad MI, Kong Q, Montero PH, Palmer FL, Xu W, De Almeida JR, Lee N, O'Sullivan B, Irish JC, Gilbert R, Ganly I, Patel SG, Goldstein DP, Morris LG. Impact of elective neck dissection on the outcome of oral squamous cell carcinomas arising in the maxillary alveolus and hard palate. Head & neck. 2016 Apr:38 Suppl 1(Suppl 1):E1688-94. doi: 10.1002/hed.24302. Epub 2015 Nov 28
[PubMed PMID: 26614119]
[34]
Os AD, Karakullukcu B, Leemans CR, Halmos GB, Roodenburg JL, Weert SV, Karagozoglu KH, Witjes MJ. Management of the clinically N0 neck in squamous cell carcinoma of the maxillary alveolus and hard palate. Head & neck. 2016 Dec:38(12):1794-1798. doi: 10.1002/hed.24511. Epub 2016 Jul 4
[PubMed PMID: 27375001]
[35]
Alonso JE, Han AY, Kuan EC, Strohl M, Clair JM, St John MA, Ryan WR, Heaton CM. The survival impact of surgical therapy in squamous cell carcinoma of the hard palate. The Laryngoscope. 2018 Sep:128(9):2050-2055. doi: 10.1002/lary.27080. Epub 2018 Feb 5
[PubMed PMID: 29399797]
[36]
Zheng Y, Xiao Z, Zhang H, She D, Lin X, Lin Y, Cao D. Differentiation between benign and malignant palatal tumors using conventional MRI: a retrospective analysis of 130 cases. Oral surgery, oral medicine, oral pathology and oral radiology. 2018 Apr:125(4):343-350. doi: 10.1016/j.oooo.2018.01.006. Epub 2018 Jan 31
[PubMed PMID: 29477604]
Level 2 (mid-level) evidence
[37]
Woolgar JA. Histological distribution of cervical lymph node metastases from intraoral/oropharyngeal squamous cell carcinomas. The British journal of oral & maxillofacial surgery. 1999 Jun:37(3):175-80
[PubMed PMID: 10454023]
[38]
Trivedi NP, Ravindran HK, Sundram S, Iyer S, Kekatpure V, Durah S, Kuriakose MA. Pathologic evaluation of sentinel lymph nodes in oral squamous cell carcinoma. Head & neck. 2010 Nov:32(11):1437-43. doi: 10.1002/hed.21345. Epub
[PubMed PMID: 20146343]
[39]
Beckhardt RN, Weber RS, Zane R, Garden AS, Wolf P, Carrillo R, Luna MA. Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. The Laryngoscope. 1995 Nov:105(11):1155-60
[PubMed PMID: 7475867]
[40]
Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern California and comparison to reports from other parts of the world. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2007 Apr:36(4):207-14
[PubMed PMID: 17391298]
Level 3 (low-level) evidence
[41]
Pires FR, Pringle GA, de Almeida OP, Chen SY. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral oncology. 2007 May:43(5):463-70
[PubMed PMID: 16979373]
Level 3 (low-level) evidence
[42]
Ganly I, Patel SG, Coleman M, Ghossein R, Carlson D, Shah JP. Malignant minor salivary gland tumors of the larynx. Archives of otolaryngology--head & neck surgery. 2006 Jul:132(7):767-70
[PubMed PMID: 16847187]
[43]
Li Q, Zhang XR, Liu XK, Liu ZM, Liu WW, Li H, Guo ZM. Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral oncology. 2012 May:48(5):456-62. doi: 10.1016/j.oraloncology.2011.12.005. Epub 2012 Jan 16
[PubMed PMID: 22248739]
[44]
Iyer NG, Kim L, Nixon IJ, Palmer F, Kraus D, Shaha AR, Shah JP, Patel SG, Ganly I. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Archives of otolaryngology--head & neck surgery. 2010 Dec:136(12):1240-7. doi: 10.1001/archoto.2010.213. Epub
[PubMed PMID: 21173374]
[45]
Tian Z, Li L, Wang L, Hu Y, Li J. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. International journal of oral and maxillofacial surgery. 2010 Mar:39(3):235-42. doi: 10.1016/j.ijom.2009.10.016. Epub 2009 Nov 29
[PubMed PMID: 19951834]
Level 2 (mid-level) evidence
[46]
Chundru NS, Amudala R, Thankappan P, Nagaraju CD. Adenoid cystic carcinoma of palate: A case report and review of literature. Dental research journal. 2013 Mar:10(2):274-8
[PubMed PMID: 23946749]
Level 3 (low-level) evidence
[47]
Yaga US, Gollamudi N, Mengji AK, Besta R, Panta P, Prakash B, Rajashekar E. Adenoid cystic carcinoma of the palate: case report and review of literature. The Pan African medical journal. 2016:24():106. doi: 10.11604/pamj.2016.24.106.8596. Epub 2016 May 31
[PubMed PMID: 27642445]
Level 3 (low-level) evidence
[48]
Eneroth CM, Hjertman L, Moberger G. Adenoid cystic carcinoma of the palate. Acta oto-laryngologica. 1968:66(3):248-60
[PubMed PMID: 4303189]
[49]
da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, de Almeida OP, Kowalski LP. Prognostic factors in head and neck adenoid cystic carcinoma. Oral oncology. 2006 Feb:42(2):139-46
[PubMed PMID: 16249115]
[50]
Werther PL, Alawi F, Lindemeyer RG. Mucoepidermoid carcinoma of the palate in adolescence. Journal of dentistry for children (Chicago, Ill.). 2015 Jan-Apr:82(1):57-61
[PubMed PMID: 25909845]
[51]
Darling MR, Schneider JW, Phillips VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral oncology. 2002 Oct:38(7):641-5
[PubMed PMID: 12167416]
[52]
Hunter JB, Smith RV, Brandwein-Gensler M. Low-grade papillary adenocarcinoma of the palate: the significance of distinguishing it from polymorphous low-grade adenocarcinoma. Head and neck pathology. 2008 Dec:2(4):316-23. doi: 10.1007/s12105-008-0082-1. Epub 2008 Sep 12
[PubMed PMID: 20614302]
[53]
Paleri V, Robinson M, Bradley P. Polymorphous low-grade adenocarcinoma of the head and neck. Current opinion in otolaryngology & head and neck surgery. 2008 Apr:16(2):163-9. doi: 10.1097/MOO.0b013e3282f70441. Epub
[PubMed PMID: 18327037]
Level 3 (low-level) evidence
[54]
Mills SE, Garland TA, Allen MS Jr. Low-grade papillary adenocarcinoma of palatal salivary gland origin. The American journal of surgical pathology. 1984 May:8(5):367-74
[PubMed PMID: 6731663]
[55]
Utsunomiya T, Yamamoto H, Kuyama K, Itami M, Asanuma K. Acinic cell carcinoma of the palate: case report and immunohistochemical observation. Archives of otolaryngology--head & neck surgery. 1999 Sep:125(9):1025-8
[PubMed PMID: 10488991]
Level 3 (low-level) evidence
[56]
Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. International journal of clinical and experimental pathology. 2012:5(8):739-53
[PubMed PMID: 23071856]
[57]
Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cancer treatment and research. 2016:167():295-320. doi: 10.1007/978-3-319-22539-5_13. Epub
[PubMed PMID: 26601869]
[58]
Postow MA, Hamid O, Carvajal RD. Mucosal melanoma: pathogenesis, clinical behavior, and management. Current oncology reports. 2012 Oct:14(5):441-8. doi: 10.1007/s11912-012-0244-x. Epub
[PubMed PMID: 22661391]
[59]
Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. Journal of the American Academy of Dermatology. 2007 May:56(5):828-34
[PubMed PMID: 17349716]
[60]
Lourenço SV, Fernandes JD, Hsieh R, Coutinho-Camillo CM, Bologna S, Sangueza M, Nico MM. Head and neck mucosal melanoma: a review. The American Journal of dermatopathology. 2014 Jul:36(7):578-87. doi: 10.1097/DAD.0000000000000035. Epub
[PubMed PMID: 24423929]
[61]
Yde SS, Sjoegren P, Heje M, Stolle LB. Mucosal Melanoma: a Literature Review. Current oncology reports. 2018 Mar 23:20(3):28. doi: 10.1007/s11912-018-0675-0. Epub 2018 Mar 23
[PubMed PMID: 29569184]
[62]
Fatahzadeh M, Schwartz RA. Oral Kaposi's sarcoma: a review and update. International journal of dermatology. 2013 Jun:52(6):666-72. doi: 10.1111/j.1365-4632.2012.05758.x. Epub
[PubMed PMID: 23679875]
[63]
Pantanowitz L, Khammissa RA, Lemmer J, Feller L. Oral HIV-associated Kaposi sarcoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2013 Mar:42(3):201-7. doi: 10.1111/j.1600-0714.2012.01180.x. Epub 2012 Jun 5
[PubMed PMID: 22672182]
[64]
Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet (London, England). 2012 Sep 1:380(9844):848-57. doi: 10.1016/S0140-6736(12)60605-9. Epub 2012 Jul 25
[PubMed PMID: 22835603]
[65]
Ugboko VI, Oginni FO, Adelusola KA, Durosinmi MA. Orofacial non-Hodgkins lymphoma in Nigerians. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2004 Nov:62(11):1347-50
[PubMed PMID: 15510355]
[66]
Mawardi H, Cutler C, Treister N. Medical management update: Non-Hodgkin lymphoma. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009 Jan:107(1):e19-33. doi: 10.1016/j.tripleo.2008.08.054. Epub
[PubMed PMID: 19101479]
[67]
Manveen JK, Subramanyam R, Harshaminder G, Madhu S, Narula R. Primary B-cell MALT lymphoma of the palate: A case report and distinction from benign lymphoid hyperplasia (pseudolymphoma). Journal of oral and maxillofacial pathology : JOMFP. 2012 Jan:16(1):97-102. doi: 10.4103/0973-029X.92982. Epub
[PubMed PMID: 22438648]
Level 3 (low-level) evidence
[68]
Kemp S, Gallagher G, Kabani S, Noonan V, O'Hara C. Oral non-Hodgkin's lymphoma: review of the literature and World Health Organization classification with reference to 40 cases. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2008 Feb:105(2):194-201
[PubMed PMID: 17604660]
Level 3 (low-level) evidence
[69]
Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncology letters. 2014 Jul:8(1):7-11
[PubMed PMID: 24959211]
Level 3 (low-level) evidence
[70]
Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA: a cancer journal for clinicians. 2015 Sep-Oct:65(5):401-21. doi: 10.3322/caac.21293. Epub 2015 Jul 27
[PubMed PMID: 26215712]
[71]
Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, Zhang L, Mitani M, Weber RS, Lippman SM, Caulin C, El-Naggar AK. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Nov 15:17(22):7003-14. doi: 10.1158/1078-0432.CCR-11-1870. Epub 2011 Oct 5
[PubMed PMID: 21976542]
Level 2 (mid-level) evidence
[72]
Brill LB 2nd, Kanner WA, Fehr A, Andrén Y, Moskaluk CA, Löning T, Stenman G, Frierson HF Jr. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 Sep:24(9):1169-76. doi: 10.1038/modpathol.2011.86. Epub 2011 May 13
[PubMed PMID: 21572406]
[73]
Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor-Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer discovery. 2016 Feb:6(2):176-87. doi: 10.1158/2159-8290.CD-15-0859. Epub 2015 Dec 2
[PubMed PMID: 26631070]
[74]
Bell D, El-Naggar AK. Molecular heterogeneity in mucoepidermoid carcinoma: conceptual and practical implications. Head and neck pathology. 2013 Mar:7(1):23-7. doi: 10.1007/s12105-013-0432-5. Epub 2013 Mar 5
[PubMed PMID: 23459841]
[75]
El-Naggar AK, Lovell M, Killary AM, Clayman GL, Batsakis JG. A mucoepidermoid carcinoma of minor salivary gland with t(11;19)(q21;p13.1) as the only karyotypic abnormality. Cancer genetics and cytogenetics. 1996 Mar:87(1):29-33
[PubMed PMID: 8646736]
[76]
Abrams AM, Melrose RJ. Acinic cell tumors of minor salivary gland origin. Oral surgery, oral medicine, and oral pathology. 1978 Aug:46(2):220-33
[PubMed PMID: 280830]
[77]
Chen SY, Brannon RB, Miller AS, White DK, Hooker SP. Acinic cell adenocarcinoma of minor salivary glands. Cancer. 1978 Aug:42(2):678-85
[PubMed PMID: 679160]
[78]
Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003 May 19:22(20):3016-23
[PubMed PMID: 12789276]
[79]
Mackintosh JA. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. Journal of theoretical biology. 2001 Jul 21:211(2):101-13
[PubMed PMID: 11419954]
[80]
López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, Mendenhall WM, Haigentz M Jr, Strojan P, Pellitteri PK, Bradford CR, Shaha AR, Hunt JL, de Bree R, Takes RP, Rinaldo A, Ferlito A. Update on primary head and neck mucosal melanoma. Head & neck. 2016 Jan:38(1):147-55. doi: 10.1002/hed.23872. Epub 2015 May 22
[PubMed PMID: 25242350]
[81]
Sheng X, Kong Y, Li Y, Zhang Q, Si L, Cui C, Chi Z, Tang B, Mao L, Lian B, Wang X, Yan X, Li S, Dai J, Guo J. GNAQ and GNA11 mutations occur in 9.5% of mucosal melanoma and are associated with poor prognosis. European journal of cancer (Oxford, England : 1990). 2016 Sep:65():156-63. doi: 10.1016/j.ejca.2016.06.019. Epub 2016 Aug 4
[PubMed PMID: 27498141]
[82]
Wu YH, Yang H, Sun A, Chen HM. Kaposi's sarcoma of the hard palate. Journal of the Formosan Medical Association = Taiwan yi zhi. 2016 Oct:115(10):883-884. doi: 10.1016/j.jfma.2016.06.009. Epub 2016 Sep 6
[PubMed PMID: 27612664]
[83]
Pratap S, Scordino TS. Molecular and cellular genetics of non-Hodgkin lymphoma: Diagnostic and prognostic implications. Experimental and molecular pathology. 2019 Feb:106():44-51. doi: 10.1016/j.yexmp.2018.11.008. Epub 2018 Nov 19
[PubMed PMID: 30465756]
[84]
Trotta BM, Pease CS, Rasamny JJ, Raghavan P, Mukherjee S. Oral cavity and oropharyngeal squamous cell cancer: key imaging findings for staging and treatment planning. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011 Mar-Apr:31(2):339-54. doi: 10.1148/rg.312105107. Epub
[PubMed PMID: 21415183]
[85]
Batsakis JG, Luna MA, el-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. The Annals of otology, rhinology, and laryngology. 1990 Dec:99(12):1007-9
[PubMed PMID: 2173892]
[86]
DeAngelis AF, Tsui A, Wiesenfeld D, Chandu A. Outcomes of patients with adenoid cystic carcinoma of the minor salivary glands. International journal of oral and maxillofacial surgery. 2011 Jul:40(7):710-4. doi: 10.1016/j.ijom.2011.02.010. Epub 2011 Mar 10
[PubMed PMID: 21396798]
[87]
Shimamoto H, Chindasombatjaroen J, Kakimoto N, Kishino M, Murakami S, Furukawa S. Perineural spread of adenoid cystic carcinoma in the oral and maxillofacial regions: evaluation with contrast-enhanced CT and MRI. Dento maxillo facial radiology. 2012 Feb:41(2):143-51. doi: 10.1259/dmfr/21825064. Epub
[PubMed PMID: 22301639]
[88]
Okahara M, Kiyosue H, Hori Y, Matsumoto A, Mori H, Yokoyama S. Parotid tumors: MR imaging with pathological correlation. European radiology. 2003 Dec:13 Suppl 4():L25-33
[PubMed PMID: 15018162]
[89]
Ikawa T, Ohkubo Y, Kitao K, Kamizaki Y, Ohbuchi M. Mucoepidermoid carcinoma of the hard palate. Auris, nasus, larynx. 1985:12(2):89-94
[PubMed PMID: 4074213]
[90]
Herd MK, Murugaraj V, Ghataura SS, Brennan PA, Anand R. Low-grade mucoepidermoid carcinoma of the palate-a previously unreported case of metastasis to the liver. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2012 Oct:70(10):2343-6. doi: 10.1016/j.joms.2011.11.019. Epub 2012 Jan 28
[PubMed PMID: 22285339]
Level 3 (low-level) evidence
[91]
González-García R, Rodríguez-Campo FJ, Muñoz-Guerra MF, Nam-Cha SH, Sastre-Pérez J, Naval-Gías L. Polymorphous low-grade adenocarcinoma of the palate: report of cases. Auris, nasus, larynx. 2005 Sep:32(3):275-80
[PubMed PMID: 15963668]
Level 3 (low-level) evidence
[92]
Gibbons D, Saboorian MH, Vuitch F, Gokaslan ST, Ashfaq R. Fine-needle aspiration findings in patients with polymorphous low grade adenocarcinoma of the salivary glands. Cancer. 1999 Feb 25:87(1):31-6
[PubMed PMID: 10096357]
[93]
Slootweg PJ, Müller H. Low-grade adenocarcinoma of the oral cavity. A comparison between the terminal duct and the papillary type. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 1987 Dec:15(6):359-64
[PubMed PMID: 2828432]
[94]
Sato S, Kawamura H, Ooya W. Acinic cell tumor of the hard palate. International journal of oral and maxillofacial surgery. 1991 Oct:20(5):271-2
[PubMed PMID: 1662249]
[95]
Suh SI, Seol HY, Kim TK, Lee NJ, Kim JH, Kim KA, Woo JS, Lee JH. Acinic cell carcinoma of the head and neck: radiologic-pathologic correlation. Journal of computer assisted tomography. 2005 Jan-Feb:29(1):121-6
[PubMed PMID: 15665697]
[96]
Ascierto PA, Accorona R, Botti G, Farina D, Fossati P, Gatta G, Gogas H, Lombardi D, Maroldi R, Nicolai P, Ravanelli M, Vanella V. Mucosal melanoma of the head and neck. Critical reviews in oncology/hematology. 2017 Apr:112():136-152. doi: 10.1016/j.critrevonc.2017.01.019. Epub 2017 Feb 13
[PubMed PMID: 28325255]
[97]
Feller L, Lemmer J, Wood NH, Jadwat Y, Raubenheimer EJ. HIV-associated oral Kaposi sarcoma and HHV-8: a review. Journal of the International Academy of Periodontology. 2007 Oct:9(4):129-36
[PubMed PMID: 17987883]
[98]
Medeiros LJ, Harmon DC, Linggood RM, Harris NL. Immunohistologic features predict clinical behavior of orbital and conjunctival lymphoid infiltrates. Blood. 1989 Nov 1:74(6):2121-9
[PubMed PMID: 2804350]
[99]
Gilliam AC, Wood GS. Cutaneous lymphoid hyperplasias. Seminars in cutaneous medicine and surgery. 2000 Jun:19(2):133-41
[PubMed PMID: 10892716]
[100]
Moore BA, Burkey BB, Netterville JL, Butcher RB 2nd, Amedee RG. Surgical management of minor salivary gland neoplasms of the palate. Ochsner journal. 2008 Winter:8(4):172-80
[PubMed PMID: 21603498]
[101]
Sarmento DJ, Morais ML, Costa AL, Silveira ÉJ. Minor intraoral salivary gland tumors: a clinical-pathological study. Einstein (Sao Paulo, Brazil). 2016 Oct-Dec:14(4):508-512. doi: 10.1590/S1679-45082016AO3749. Epub
[PubMed PMID: 28076598]
[102]
Zhang WB, Peng X. Cervical metastases of oral maxillary squamous cell carcinoma: A systematic review and meta-analysis. Head & neck. 2016 Apr:38 Suppl 1():E2335-42. doi: 10.1002/hed.24274. Epub 2016 Feb 18
[PubMed PMID: 26890607]
Level 1 (high-level) evidence
[103]
Pushpanjali M, Sujata DN, Subramanyam SB, Jyothsna M. Adenoid cystic carcinoma: An unusual presentation. Journal of oral and maxillofacial pathology : JOMFP. 2014 May:18(2):286-90. doi: 10.4103/0973-029X.140796. Epub
[PubMed PMID: 25328314]
[105]
Ritwik P, Cordell KG, Brannon RB. Minor salivary gland mucoepidermoid carcinoma in children and adolescents: a case series and review of the literature. Journal of medical case reports. 2012 Jul 3:6():182. doi: 10.1186/1752-1947-6-182. Epub 2012 Jul 3
[PubMed PMID: 22759529]
Level 2 (mid-level) evidence
[106]
Seitz O, Chambron-Pinho N, Middendorp M, Sader R, Mack M, Vogl TJ, Bisdas S. 18F-Fluorodeoxyglucose-PET/CT to evaluate tumor, nodal disease, and gross tumor volume of oropharyngeal and oral cavity cancer: comparison with MR imaging and validation with surgical specimen. Neuroradiology. 2009 Oct:51(10):677-86. doi: 10.1007/s00234-009-0586-8. Epub
[PubMed PMID: 19727695]
Level 1 (high-level) evidence
[107]
Vidiri A, Guerrisi A, Pellini R, Manciocco V, Covello R, Mattioni O, Guerrisi I, Di Giovanni S, Spriano G, Crecco M. Multi-detector row computed tomography (MDCT) and magnetic resonance imaging (MRI) in the evaluation of the mandibular invasion by squamous cell carcinomas (SCC) of the oral cavity. Correlation with pathological data. Journal of experimental & clinical cancer research : CR. 2010 Jun 17:29(1):73. doi: 10.1186/1756-9966-29-73. Epub 2010 Jun 17
[PubMed PMID: 20565737]
[108]
Gu DH, Yoon DY, Park CH, Chang SK, Lim KJ, Seo YL, Yun EJ, Choi CS, Bae SH. CT, MR, (18)F-FDG PET/CT, and their combined use for the assessment of mandibular invasion by squamous cell carcinomas of the oral cavity. Acta radiologica (Stockholm, Sweden : 1987). 2010 Dec:51(10):1111-9. doi: 10.3109/02841851.2010.520027. Epub 2010 Oct 7
[PubMed PMID: 20929295]
[109]
von Stempel C, Morley S, Beale T, Otero S. Imaging of palatal lumps. Clinical radiology. 2017 Feb:72(2):97-107. doi: 10.1016/j.crad.2016.10.007. Epub 2016 Dec 14
[PubMed PMID: 27986264]
[110]
Weber AL, Bui C, Kaneda T. Malignant tumors of the mandible and maxilla. Neuroimaging clinics of North America. 2003 Aug:13(3):509-24
[PubMed PMID: 14631688]
[111]
Krabbe CA, Balink H, Roodenburg JL, Dol J, de Visscher JG. Performance of 18F-FDG PET/contrast-enhanced CT in the staging of squamous cell carcinoma of the oral cavity and oropharynx. International journal of oral and maxillofacial surgery. 2011 Nov:40(11):1263-70. doi: 10.1016/j.ijom.2011.06.023. Epub 2011 Aug 6
[PubMed PMID: 21824748]
[112]
Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, Wang HM, Wong HF. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005 Jul:46(7):1136-43
[PubMed PMID: 16000282]
[113]
Ozer E, Naiboğlu B, Meacham R, Ryoo C, Agrawal A, Schuller DE. The value of PET/CT to assess clinically negative necks. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2012 Nov:269(11):2411-4. doi: 10.1007/s00405-012-1926-6. Epub 2012 Jan 15
[PubMed PMID: 22249837]
[114]
Yang Z, Deng R, Sun G, Huang X, Tang E. Cervical metastases from squamous cell carcinoma of hard palate and maxillary alveolus: a retrospective study of 10 years. Head & neck. 2014 Jul:36(7):969-75. doi: 10.1002/hed.23398. Epub 2013 Sep 18
[PubMed PMID: 23733304]
Level 2 (mid-level) evidence
[115]
Copelli C, Bianchi B, Ferrari S, Ferri A, Sesenna E. Malignant tumors of intraoral minor salivary glands. Oral oncology. 2008 Jul:44(7):658-63
[PubMed PMID: 17996484]
[116]
Milgrom SA, Yahalom J. Indolent non-Hodgkin lymphoma primarily involving the hard palate: outcome following radiotherapy. Leukemia & lymphoma. 2013 Jun:54(6):1208-11. doi: 10.3109/10428194.2012.741232. Epub 2012 Nov 20
[PubMed PMID: 23083063]
[117]
Brown JS, Magennis P, Rogers SN, Cawood JI, Howell R, Vaughan ED. Trends in head and neck microvascular reconstructive surgery in Liverpool (1992-2001). The British journal of oral & maxillofacial surgery. 2006 Oct:44(5):364-70
[PubMed PMID: 16169640]
[118]
Sahoo NK, Desai AP, Roy ID, Kulkarni V. Oro-Nasal Communication. The Journal of craniofacial surgery. 2016 Sep:27(6):e529-33. doi: 10.1097/SCS.0000000000002815. Epub
[PubMed PMID: 27607130]
[119]
Cannon RB, Sowder JC, Buchmann LO, Hunt JP, Hitchcock YJ, Lloyd S, Grossman KF, Monroe MM. Increasing use of nonsurgical therapy in advanced-stage oral cavity cancer: A population-based study. Head & neck. 2017 Jan:39(1):82-91. doi: 10.1002/hed.24542. Epub 2016 Sep 19
[PubMed PMID: 27641220]
[120]
Yang X, Song X, Chu W, Li L, Ma L, Wu Y. Clinicopathological Characteristics and Outcome Predictors in Squamous Cell Carcinoma of the Maxillary Gingiva and Hard Palate. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2015 Jul:73(7):1429-36. doi: 10.1016/j.joms.2014.12.034. Epub 2015 Jan 10
[PubMed PMID: 25869748]
[121]
Eskander A, Givi B, Gullane PJ, Irish J, Brown D, Gilbert RW, Hope A, Weinreb I, Xu W, Goldstein DP. Outcome predictors in squamous cell carcinoma of the maxillary alveolus and hard palate. The Laryngoscope. 2013 Oct:123(10):2453-8. doi: 10.1002/lary.24079. Epub 2013 Apr 1
[PubMed PMID: 23553191]
[122]
Hicks WL Jr, Loree TR, Garcia RI, Maamoun S, Marshall D, Orner JB, Bakamjian VY, Shedd DP. Squamous cell carcinoma of the floor of mouth: a 20-year review. Head & neck. 1997 Aug:19(5):400-5
[PubMed PMID: 9243267]
[123]
Jakobsson PA, Blanck C, Eneroth CM. Mucoepidermoid carcinoma of the parotid gland. Cancer. 1968 Jul:22(1):111-24
[PubMed PMID: 4298176]
[124]
Liu XK, Zeng ZY, Chen FJ, Guo ZM, Xu GP, Yang AK, Zhang Q. [Effectiveness evaluation and prognostic factor analysis in patients with minor salivary gland carcinoma of the hard palate]. Ai zheng = Aizheng = Chinese journal of cancer. 2003 Oct:22(10):1088-92
[PubMed PMID: 14558958]
[125]
Kumar M, Stivaros N, Barrett AW, Thomas GJ, Bounds G, Newman L. Polymorphous low-grade adenocarcinoma--a rare and aggressive entity in adolescence. The British journal of oral & maxillofacial surgery. 2004 Jun:42(3):195-9
[PubMed PMID: 15121262]
[126]
Xiao R, Sethi RKV, Feng AL, Fontanarosa JB, Deschler DG. The role of elective neck dissection in patients with adenoid cystic carcinoma of the head and neck. The Laryngoscope. 2019 Sep:129(9):2094-2104. doi: 10.1002/lary.27814. Epub 2019 Jan 22
[PubMed PMID: 30667061]
[127]
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK, Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2004 May 6:350(19):1937-44
[PubMed PMID: 15128893]
[128]
Qing J, Zhang Q, Wei MW, Guo ZM. [Prognostic analysis of adenoid cystic carcinoma of major salivary glands of 64 cases]. Ai zheng = Aizheng = Chinese journal of cancer. 2006 Sep:25(9):1138-43
[PubMed PMID: 16965658]
Level 2 (mid-level) evidence
[129]
Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck--a 20 years experience. International journal of oral and maxillofacial surgery. 2004 Jan:33(1):25-31
[PubMed PMID: 14690656]
[130]
Bai S, Clubwala R, Adler E, Sarta C, Schiff B, Smith RV, Gnepp DR, Brandwein-Gensler M. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head and neck pathology. 2013 Jun:7(2):105-12. doi: 10.1007/s12105-012-0405-0. Epub 2012 Oct 19
[PubMed PMID: 23080318]
[131]
Pinto PX, Coleman N. Regional metastasis in polymorphous low grade adenocarcinoma. Report of a case. International journal of oral and maxillofacial surgery. 1997 Dec:26(6):447-9
[PubMed PMID: 9418148]
Level 3 (low-level) evidence
[132]
Colmenero CM, Patron M, Burgueño M, Sierra I. Polymorphous low-grade adenocarcinoma of the oral cavity: a report of 14 cases. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1992 Jun:50(6):595-600
[PubMed PMID: 1593320]
Level 3 (low-level) evidence
[133]
Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. The American journal of surgical pathology. 2000 Oct:24(10):1319-28
[PubMed PMID: 11023093]
Level 3 (low-level) evidence
[134]
Perzin KH, LiVolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979 Oct:44(4):1434-57
[PubMed PMID: 498020]
[135]
Wushou A, Hou J, Zhao YJ, Miao XC. Postoperative adjuvant radiotherapy improves loco-regional recurrence of head and neck mucosal melanoma. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2015 May:43(4):553-8. doi: 10.1016/j.jcms.2015.02.011. Epub 2015 Feb 21
[PubMed PMID: 25797388]
[136]
Di Lorenzo S, Corradino B, Panzarella V. Non-Hodgkin B-cell lymphoma involving the palate. The Lancet. Oncology. 2018 May:19(5):e275. doi: 10.1016/S1470-2045(17)30606-X. Epub
[PubMed PMID: 29726392]
[137]
Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head & neck. 2005 Oct:27(10):843-50
[PubMed PMID: 16161069]
Level 2 (mid-level) evidence
[138]
Creagan ET, Woods JE, Schutt AJ, O'Fallon JR. Cyclophosphamide, adriamycin, and cis-diamminedichloroplatinum (II) in the treatment of advanced nonsquamous cell head and neck cancer. Cancer. 1983 Dec 1:52(11):2007-10
[PubMed PMID: 6684986]
[139]
Vander Poorten VL, Balm AJ, Hilgers FJ, Tan IB, Keus RB, Hart AA. Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer. 2000 Sep 15:89(6):1195-204
[PubMed PMID: 11002213]
[140]
Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas - a review. Oncotarget. 2017 Jan 17:8(3):3946-3956. doi: 10.18632/oncotarget.13952. Epub
[PubMed PMID: 27992367]
[141]
Mücke T, Tannapfel A, Kesting MR, Wagenpfeil S, Robitzky LK, Wolff KD, Hölzle F. Adenoid cystic carcinomas of minor salivary glands. Auris, nasus, larynx. 2010 Oct:37(5):615-20. doi: 10.1016/j.anl.2010.02.001. Epub 2010 Feb 23
[PubMed PMID: 20181451]
[142]
Wu CF, Wu CS, Yu WW, Huang MY. Complete response of huge buccal malignant melanoma in an octogenarian patient to arterial chemotherapy. Head & neck. 2015 Oct:37(10):E134-8. doi: 10.1002/hed.23941. Epub 2015 Jun 22
[PubMed PMID: 25521097]
[143]
Umeda M, Murata M, Suzuki H, Yanagida T, Shibuya Y, Komori T. A case of malignant melanoma of the oral cavity alive with liver metastasis for a long period with administration of a biologic response modifier, OK432. The Kobe journal of medical sciences. 2010 Oct 21:56(3):E140-7
[PubMed PMID: 21063154]
Level 3 (low-level) evidence
[144]
Postow MA, Luke JJ, Bluth MJ, Ramaiya N, Panageas KS, Lawrence DP, Ibrahim N, Flaherty KT, Sullivan RJ, Ott PA, Callahan MK, Harding JJ, D'Angelo SP, Dickson MA, Schwartz GK, Chapman PB, Gnjatic S, Wolchok JD, Hodi FS, Carvajal RD. Ipilimumab for patients with advanced mucosal melanoma. The oncologist. 2013 Jun:18(6):726-32. doi: 10.1634/theoncologist.2012-0464. Epub 2013 May 28
[PubMed PMID: 23716015]
[145]
Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ, Gangadhar TC, Salama AK, Clark V, Burias C, Puzanov I, Atkins MB, Algazi AP, Ribas A, Wolchok JD, Postow MA. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016 Nov 15:122(21):3354-3362. doi: 10.1002/cncr.30259. Epub 2016 Aug 17
[PubMed PMID: 27533633]
[146]
Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017 Mar:67(2):122-137. doi: 10.3322/caac.21389. Epub 2017 Jan 27
[PubMed PMID: 28128848]
[147]
Gilain L, Houette A, Montalban A, Mom T, Saroul N. Mucosal melanoma of the nasal cavity and paranasal sinuses. European annals of otorhinolaryngology, head and neck diseases. 2014 Dec:131(6):365-369. doi: 10.1016/j.anorl.2013.11.004. Epub 2014 Jun 3
[PubMed PMID: 24906226]
[148]
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin's Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, Southwest Oncology Group, United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Sep 20:32(27):3059-68
[PubMed PMID: 25113753]
[149]
Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head & neck. 2011 Jun:33(6):824-30. doi: 10.1002/hed.21547. Epub 2010 Oct 14
[PubMed PMID: 20949448]
[150]
Andersen PE, Cambronero E, Shaha AR, Shah JP. The extent of neck disease after regional failure during observation of the N0 neck. American journal of surgery. 1996 Dec:172(6):689-91
[PubMed PMID: 8988679]
[151]
Bradley PJ. Adenoid cystic carcinoma evaluation and management: progress with optimism! Current opinion in otolaryngology & head and neck surgery. 2017 Apr:25(2):147-153. doi: 10.1097/MOO.0000000000000347. Epub
[PubMed PMID: 28106659]
Level 3 (low-level) evidence
[152]
Srivastava AC, Barpande SR, Bhavthankar JD, Mandale MS. Adenoid cystic carcinoma of palate: Report of a solid variant. Journal of oral and maxillofacial pathology : JOMFP. 2018 Jan:22(Suppl 1):S65-S68. doi: 10.4103/jomfp.JOMFP_5_16. Epub
[PubMed PMID: 29491609]
[153]
Hashimoto S, Takahashi H, Okamoto M, Yao K, Nakayama M, Makoshi T, Iwabuchi K. Prognostic factors of head and neck adenoid cystic carcinoma: quantitative morphological analysis of 19 cases. Acta oto-laryngologica. Supplementum. 2002:(547):93-6
[PubMed PMID: 12212605]
Level 3 (low-level) evidence
[154]
Ozdemir C, Karacetin D, Tuna S, Karadeniz A. Treatment and clinicopathologic predictors for adenoid cystic carcinomas of the head and neck. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2011 Jan-Mar:16(1):123-6
[PubMed PMID: 21674862]
[155]
Khafif A, Anavi Y, Haviv J, Fienmesser R, Calderon S, Marshak G. Adenoid cystic carcinoma of the salivary glands: a 20-year review with long-term follow-up. Ear, nose, & throat journal. 2005 Oct:84(10):662, 664-7
[PubMed PMID: 16382750]
[156]
Suei Y, Tanimoto K, Taguchi A, Wada T. Radiographic evaluation of bone invasion of adenoid cystic carcinoma in the oral and maxillofacial region. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1994 Aug:52(8):821-6
[PubMed PMID: 8040735]
[157]
Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. International journal of oral and maxillofacial surgery. 1997 Dec:26(6):435-9
[PubMed PMID: 9418145]
[159]
Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary gland origin. A clinicopathologic study of 367 cases. American journal of surgery. 1978 Oct:136(4):461-8
[PubMed PMID: 707726]
Level 3 (low-level) evidence
[160]
Ryan JT, El-Naggar AK, Huh W, Hanna EY, Weber RS, Kupferman ME. Primacy of surgery in the management of mucoepidermoid carcinoma in children. Head & neck. 2011 Dec:33(12):1769-73. doi: 10.1002/hed.21675. Epub 2011 Jan 31
[PubMed PMID: 21284057]
[161]
Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head & neck. 2014 Feb:36(2):158-63. doi: 10.1002/hed.23256. Epub 2013 Jun 14
[PubMed PMID: 23765800]
[162]
Kimple AJ, Austin GK, Shah RN, Welch CM, Funkhouser WK, Zanation AM, Shockley WW. Polymorphous low-grade adenocarcinoma: a case series and determination of recurrence. The Laryngoscope. 2014 Dec:124(12):2714-9. doi: 10.1002/lary.24788. Epub 2014 Sep 17
[PubMed PMID: 25229805]
Level 2 (mid-level) evidence
[163]
Patel TD, Vazquez A, Marchiano E, Park RC, Baredes S, Eloy JA. Polymorphous low-grade adenocarcinoma of the head and neck: A population-based study of 460 cases. The Laryngoscope. 2015 Jul:125(7):1644-9. doi: 10.1002/lary.25266. Epub 2015 Apr 15
[PubMed PMID: 25877006]
Level 3 (low-level) evidence
[164]
Mitchell DA, Eveson JW, Ord RA. Polymorphous low-grade adenocarcinoma of minor salivary glands--a report of three cases. The British journal of oral & maxillofacial surgery. 1989 Dec:27(6):494-500
[PubMed PMID: 2688740]
Level 3 (low-level) evidence
[165]
Scherl C, Kato MG, Erkul E, Graboyes EM, Nguyen SA, Chi AC, Morgan PF, Day TA. Outcomes and prognostic factors for parotid acinic cell Carcinoma: A National Cancer Database study of 2362 cases. Oral oncology. 2018 Jul:82():53-60. doi: 10.1016/j.oraloncology.2018.05.002. Epub 2018 May 15
[PubMed PMID: 29909902]
Level 3 (low-level) evidence
[166]
Wushou A, Zhao YJ. The management and site-specific prognostic factors of primary oral mucosal malignant melanoma. The Journal of craniofacial surgery. 2015 Mar:26(2):430-4. doi: 10.1097/SCS.0000000000001328. Epub
[PubMed PMID: 25668115]
[167]
Jones JL, Hanson DL, Dworkin MS, Jaffe HW. Incidence and trends in Kaposi's sarcoma in the era of effective antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999). 2000 Jul 1:24(3):270-4
[PubMed PMID: 10969352]
[168]
Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, Virgo P, Pfeiffer RM. Survival after cancer diagnosis in persons with AIDS. Journal of acquired immune deficiency syndromes (1999). 2005 Jul 1:39(3):293-9
[PubMed PMID: 15980688]
[169]
MacDermed D, Thurber L, George TI, Hoppe RT, Le QT. Extranodal nonorbital indolent lymphomas of the head and neck: relationship between tumor control and radiotherapy. International journal of radiation oncology, biology, physics. 2004 Jul 1:59(3):788-95
[PubMed PMID: 15183482]
[170]
Gospodarowicz MK, Bush RS, Brown TC, Chua T. Prognostic factors in nodular lymphomas: a multivariate analysis based on the Princess Margaret Hospital experience. International journal of radiation oncology, biology, physics. 1984 Apr:10(4):489-97
[PubMed PMID: 6725039]
[171]
Pendlebury S, el Awadi M, Ashley S, Brada M, Horwich A. Radiotherapy results in early stage low grade nodal non-Hodgkin's lymphoma. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1995 Sep:36(3):167-71
[PubMed PMID: 8532901]
[172]
van der Waal RI, Huijgens PC, van der Valk P, van der Waal I. Characteristics of 40 primary extranodal non-Hodgkin lymphomas of the oral cavity in perspective of the new WHO classification and the International Prognostic Index. International journal of oral and maxillofacial surgery. 2005 Jun:34(4):391-5
[PubMed PMID: 16053848]
Level 3 (low-level) evidence
[173]
Hermans J, Krol AD, van Groningen K, Kluin PM, Kluin-Nelemans JC, Kramer MH, Noordijk EM, Ong F, Wijermans PW. International Prognostic Index for aggressive non-Hodgkin's lymphoma is valid for all malignancy grades. Blood. 1995 Aug 15:86(4):1460-3
[PubMed PMID: 7632953]
[174]
Cattaneo C, Facchetti F, Re A, Borlenghi E, Majorana A, Bardellini E, Casari S, Tucci A, Conti G, Rossi G. Oral cavity lymphomas in immunocompetent and human immunodeficiency virus infected patients. Leukemia & lymphoma. 2005 Jan:46(1):77-81
[PubMed PMID: 15621784]
[175]
Kolokythas A. Long-term surgical complications in the oral cancer patient: a comprehensive review. Part I. Journal of oral & maxillofacial research. 2010:1(3):e1. doi: 10.5037/jomr.2010.1301. Epub 2010 Oct 1
[PubMed PMID: 24421971]
[176]
Pauloski BR, Logemann JA, Rademaker AW, McConnel FM, Heiser MA, Cardinale S, Shedd D, Lewin J, Baker SR, Graner D. Speech and swallowing function after anterior tongue and floor of mouth resection with distal flap reconstruction. Journal of speech and hearing research. 1993 Apr:36(2):267-76
[PubMed PMID: 8487519]
[177]
Curtis DA, Plesh O, Miller AJ, Curtis TA, Sharma A, Schweitzer R, Hilsinger RL, Schour L, Singer M. A comparison of masticatory function in patients with or without reconstruction of the mandible. Head & neck. 1997 Jul:19(4):287-96
[PubMed PMID: 9213107]
[178]
Kim DD, Ord RA. Complications in the treatment of head and neck cancer. Oral and maxillofacial surgery clinics of North America. 2003 May:15(2):213-27
[PubMed PMID: 18088676]
[179]
Schauber MD, Fontenelle LJ, Solomon JW, Hanson TL. Cranial/cervical nerve dysfunction after carotid endarterectomy. Journal of vascular surgery. 1997 Mar:25(3):481-7
[PubMed PMID: 9081129]
[180]
Hey J, Setz J, Gerlach R, Janich M, Hildebrandt G, Vordermark D, Gernhardt CR, Kuhnt T. Parotid gland-recovery after radiotherapy in the head and neck region--36 months follow-up of a prospective clinical study. Radiation oncology (London, England). 2011 Sep 27:6():125. doi: 10.1186/1748-717X-6-125. Epub 2011 Sep 27
[PubMed PMID: 21951317]
[181]
Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, Adab F, Jefferies SJ, Scrase C, Yap BK, A'Hern RP, Sydenham MA, Emson M, Hall E, PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. The Lancet. Oncology. 2011 Feb:12(2):127-36. doi: 10.1016/S1470-2045(10)70290-4. Epub 2011 Jan 12
[PubMed PMID: 21236730]
Level 1 (high-level) evidence
[182]
Sher DJ, Thotakura V, Balboni TA, Norris CM Jr, Haddad RI, Posner MR, Lorch J, Goguen LA, Annino DJ, Tishler RB. Treatment of oral cavity squamous cell carcinoma with adjuvant or definitive intensity-modulated radiation therapy. International journal of radiation oncology, biology, physics. 2011 Nov 15:81(4):e215-22. doi: 10.1016/j.ijrobp.2011.02.023. Epub 2011 Apr 29
[PubMed PMID: 21531515]
[183]
Gomez DR, Zhung JE, Gomez J, Chan K, Wu AJ, Wolden SL, Pfister DG, Shaha A, Shah JP, Kraus DH, Wong RJ, Lee NY. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. International journal of radiation oncology, biology, physics. 2009 Mar 15:73(4):1096-103. doi: 10.1016/j.ijrobp.2008.05.024. Epub 2008 Aug 15
[PubMed PMID: 18707827]
[184]
Kuhnt T, Stang A, Wienke A, Vordermark D, Schweyen R, Hey J. Potential risk factors for jaw osteoradionecrosis after radiotherapy for head and neck cancer. Radiation oncology (London, England). 2016 Jul 30:11():101. doi: 10.1186/s13014-016-0679-6. Epub 2016 Jul 30
[PubMed PMID: 27473433]
[185]
Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. The journal of supportive oncology. 2007 Oct:5(9 Suppl 4):13-21
[PubMed PMID: 18046994]
[186]
PDQ Supportive and Palliative Care Editorial Board. Oral Complications of Cancer Therapies (PDQ®): Patient Version. PDQ Cancer Information Summaries. 2002:():
[PubMed PMID: 26389169]
[187]
Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, Popplewell L, Maghami E. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA: a cancer journal for clinicians. 2012 Nov-Dec:62(6):400-22. doi: 10.3322/caac.21157. Epub 2012 Sep 12
[PubMed PMID: 22972543]
[188]
Nagler RM, Nagler A. Salivary gland involvement in graft-versus-host disease: the underlying mechanism and implicated treatment. The Israel Medical Association journal : IMAJ. 2004 Mar:6(3):167-72
[PubMed PMID: 15055275]
[189]
Casares C, Ramírez-Camacho R, Trinidad A, Roldán A, Jorge E, García-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2012 Dec:269(12):2455-9. doi: 10.1007/s00405-012-2029-0. Epub 2012 May 15
[PubMed PMID: 22584749]
Level 3 (low-level) evidence
[190]
Rentschler GJ, Mann MB. The effects of glossectomy on intelligibility of speech and oral perceptual discrimination. Journal of oral surgery (American Dental Association : 1965). 1980 May:38(5):348-54
[PubMed PMID: 6928933]
[191]
Brands MT, Brennan PA, Verbeek ALM, Merkx MAW, Geurts SME. Follow-up after curative treatment for oral squamous cell carcinoma. A critical appraisal of the guidelines and a review of the literature. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018 May:44(5):559-565. doi: 10.1016/j.ejso.2018.01.004. Epub 2018 Jan 12
[PubMed PMID: 29433990]
[192]
van der Haring IS, Schaapveld MS, Roodenburg JL, de Bock GH. Second primary tumours after a squamous cell carcinoma of the oral cavity or oropharynx using the cumulative incidence method. International journal of oral and maxillofacial surgery. 2009 Apr:38(4):332-8. doi: 10.1016/j.ijom.2008.12.015. Epub 2009 Feb 4
[PubMed PMID: 19196493]