Introduction
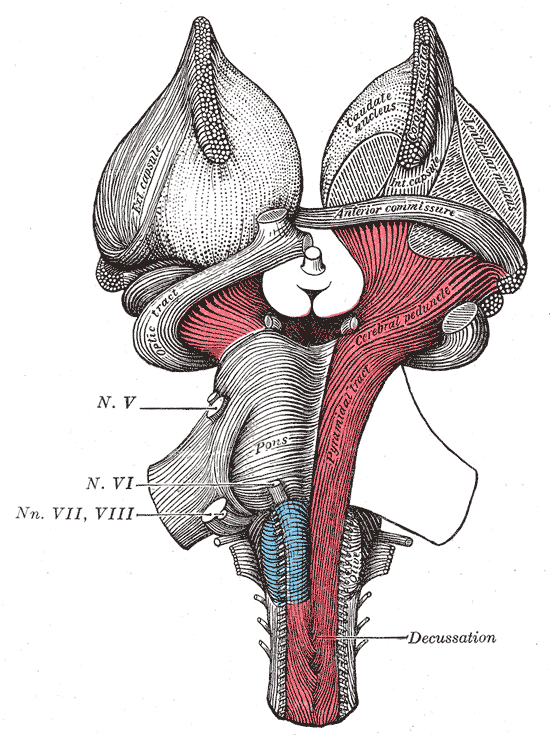
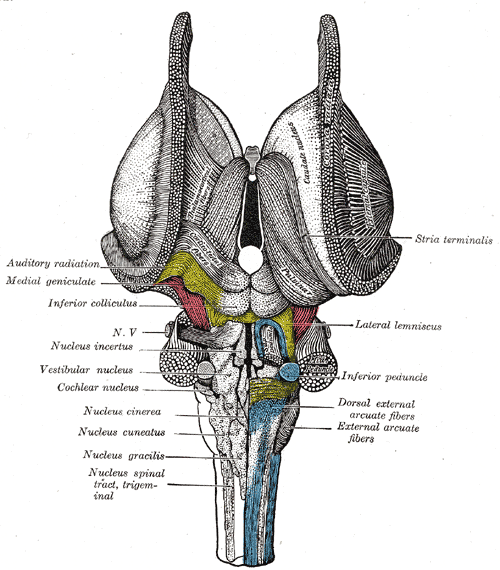
The vestibular nuclei are located in the medulla and pons of the hindbrain (see Image. The Hindbrain or Rhomencephalon, Ventral View). It is a complex composed of 4 major nuclei that integrate information from the primary vestibular afferents, contralateral nuclei, somatosensory organs, and the cerebellum. The vestibular complex projects fibers to the contralateral vestibular nuclei, spinal cord, cerebellum, thalamus, and the motor nuclei of the extraocular muscles. The major vestibular nuclei are highly interconnected with many overlapping functions. It is essential in maintaining equilibrium, posture, head position, and clear vision with movement (see Image. The Hindbrain or Rhomencephalon, Dorsal View). This article discusses the 4 major nuclei's main structural, functional, and nervous connections.
Structure and Function
The vestibular nucleus spans from the rostral medulla to the caudal pons. It is located bilaterally along the floor of the fourth ventricle and is lateral to the sulcus limitans.[1][2] The complex extends this length in 2 major columns, medial and lateral.
The vestibular complex subdivides into 4 major nuclei:
- Lateral vestibular nucleus (LVN)
- Medial vestibular nucleus (MVN)
- Superior vestibular nucleus (SVN)
- Descending (inferior) vestibular nucleus (DVN)
LVN
The LVN, also known as Deiter’s nucleus, extends along the lateral column of the vestibular complex. The SVN, MVN, and DVN border it. Lateral to the LVN are the sensory fibers of CN VIII.[3] It is distinguishable from the other nuclei by its giant cells in the dorsocaudal region and intermediate-sized cells in the rostroventral region.[2] The LVN aids the vestibulospinal reflex to maintain proper posture and balance via the limbs' paravertebral and proximal extensor muscles.[1]
MVN
The MVN, also known as the nucleus of Schwalbe, is the largest of the 4 nuclei in total cell volume and, unlike the others, runs mostly in the medial column.[3] It is bordered by the SVN and DVN, posteriorly by the fourth ventricle, and inferiorly by the dorsal motor vagal and hypoglossal nuclei. The MVN has a dorsal collection of small parvocellular neurons and a ventral collection of larger magnocellular neurons.[2] It mediates the vestibulo-ocular reflex (VOR) along with the SVN, which ensures clear vision by rotating the eyes opposite to the head during horizontal rotation.[1] For example, if the head is turned quickly to the right, both eyes turn toward the left. It also controls head and neck movements to maintain balance via the medial vestibulospinal tract.[4]
SVN
The SVN, also known as the nucleus of Bechterew, extends along the lateral column in an elongated, elliptical form. It is the most rostral of the vestibular nuclei. It is bordered ventral-laterally by the LVN, dorsally by the superior cerebellar peduncle, and medially by the fourth ventricle. Additionally, it merges ventrally with the trigeminal nucleus.[3] This elliptical-shaped nucleus has a uniform distribution of medium-sized cells. It functions to aid in coordination and postural adjustments, as well as related eye movements. It appears to play a role in the conscious perception of gravity and movement.[2]
DVN
The DVN is the most caudal of the nuclei and is neighbored superiorly by the LVN, medially by the MVN, posteriorly by the lower end of the fourth ventricle, and anteriorly by the reticular formation.[3] On microscopic examination, the DVN seems less densely populated than the other nuclei due to its characteristic longitudinal fiber bundles.[2] It receives information about head tilt and gravity, affecting blood flow, respiratory rate, and heart rate upon standing.[2]
Embryology
The nervous system develops from the ectoderm of the trilaminar embryo. From there, the neural plate folds and forms the neural tube. The rostral neural tube divides and forms the 3 primary vesicles (prosencephalon, mesencephalon, and rhombencephalon). The rhombencephalon (or hindbrain) divides into the metencephalon (pons and cerebellum) and the myelencephalon (medulla oblongata). The vestibular nuclei develop within both the metencephalon and the myelencephalon.[5]
The developing hindbrain creates 2 longitudinal columns of neurons that receive fibers from the vestibular part of the ear. These longitudinal columns subdivide into functionally specified rhombomeres (the transverse boundaries) derived from the rhombic lip. The rhombic lip is the source of migratory cell populations that give rise to the hindbrain's major dorsal and ventral nuclei. Vestibular nuclei form from several rhombomeres and are highly analogous across vertebrates, from fish to humans.[6][7]
Blood Supply and Lymphatics
The primary vascular supply to the vestibular nucleus is the posterior inferior cerebellar artery (PICA), a branch of the vertebral artery.[8]
Nerves
Vestibular nuclei interconnect via commissural projections with their homologous and nonhomologous vestibular nuclei; this allows for comparing activity on both sides.[9] All 4 of the major vestibular nuclei receive input from the entire or parts of the peripheral vestibular apparatus via the vestibular portion of cranial nerve (CN) VIII, the vestibulocochlear nerve.[10]
LVN
The LVN receives input from the vestibulocerebellum and all parts of the vestibular apparatus. Fibers from the vestibulocerebellum, also known as the flocculonodular lobe, convey head tilt and gravity information.[10] Efferent fibers from the LVN primarily regulate the lateral vestibulospinal tract, with lesser projections from the DVN. These fibers descend via the ipsilateral ventral horn of the spinal cord and extend the length of the cord, providing input to motor interneurons for the extensor muscles of the back and extremities for their respective side. The lateral vestibulospinal tract has excitatory effects on extensor motor neurons and some inhibitory effects on flexor motor neurons.[11]
MVN
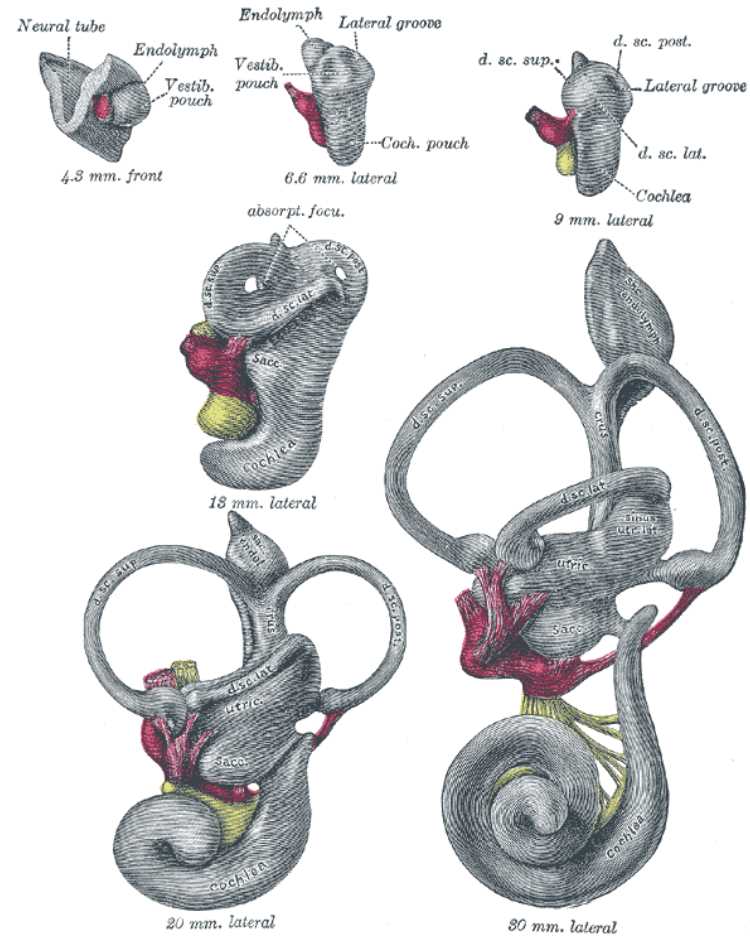
The MVN primarily receives afferent fibers from the lateral semicircular canals (see Image. Vestibular System, Semicircular Canals). Ascending fibers leave the MVN and cross the midline via the medial longitudinal fasciculus (MLF). They then innervate the contralateral paramedian pontine reticular formation (PPRF) and contralateral motor nuclei of the extraocular muscles (CN III, IV, and VI). This pathway mediates the VOR, important for clear vision during vertical and rotational movements.[4][12] Additionally, fibers that make up the medial vestibulospinal tract primarily originate from the MVN. From there, these fibers descend via the ipsilateral ventral horn of the spinal cord. They terminate in the cervical and upper thoracic spinal cord levels and provide input to motor interneurons.[11] Lastly, the MVN projects several parts of the cerebellum, including the flocculonodular lobe and the anterior and posterior vermis.[2]
SVN
The SVN receives afferent information from the superior and posterior semicircular canals. Efferent fibers from the SVN combine with fibers from the DVN to travel to the fastigial nuclei within the flocculonodular lobe of the cerebellum. The flocculonodular lobe is involved in balance maintenance and is necessary for adaptation to reflexive eye movements. The SVN also sends fibers to the nuclei of the thalamus.[2]
DVN
The DVN receives afferent fibers from the inner ear's otolith organs (the utricle and saccule). The DVN has projections to the other 3 vestibular nuclei and the cerebellum.[1] Descending projections from the DVN and MVN modulate vestibulo-sympathetic reflexes, which affect blood flow, respiratory rate, and heart rate. The medial and lateral vestibulospinal tracts also have fibers originating in the DVM.[2]
Muscles
The vestibular nuclei have terminal effects on the extraocular and postural muscles of the back, neck, and extremities.[2]
Physiologic Variants
The vestibular apparatus and vestibular nuclei have remained highly unchanged across vertebrates.[7]
Surgical Considerations
During cochlear implantation, a procedure used to restore hearing in patients with severe hearing loss, the cochlea and vestibular organs may be damaged. This damage can lead to vestibular symptoms of spontaneous nystagmus and vertigo. In time, the activity of the vestibular nucleus has shown correlations with increased compensation and plasticity, with a spontaneous decrease in central vestibular symptoms. Therefore, vestibular function testing may be important before the procedure, and careful surgery is considered in patients with functional vestibular organs.[13]
Clinical Significance
Acute Unilateral Vestibular Loss
While the cause of acute vestibular dysfunction correlates with the reactivation of a viral infection, it is unknown. However, unilateral loss of function of the vestibular nucleus is the most severe condition within the vestibular apparatus. The decreased firing rate of the compromised side causes a permanent head rotation to the healthy side, causing a slow-phase drift of the eyes toward the side of the lesion, with a rapid quick-phase eye movement back towards the healthy side.
Clinically, this leads to horizontal-torsional nystagmus, rotational vertigo, and postural imbalance. The direction of the quick phase characterizes the direction of the nystagmus. For example, a leftward quick-phase would be left-beating nystagmus. The imbalance and characteristic spontaneous nystagmus resolve within days to weeks due to restoring activity on the lesioned side.[14][15]
Wallenberg Syndrome
Wallenberg syndrome, or lateral medullary syndrome, is due to an occlusion of the vertebral artery or the posterior inferior cerebellar artery (PICA). The PICA supplies the medial portion of the posterior cerebellum and the posterolateral medulla, which includes the vestibular complex.[16] It typically occurs in a patient with hypertension, diabetes, or smoking history. Following ischemia of the vestibular nuclei, the patient usually presents with vertigo, ipsilateral nystagmus, nausea, and vomiting. Vertigo is the sensation of rotation or movement of the body or environment, and patients commonly describe it as the feeling that the room is spinning.
The involvement of the cerebellum leads to ipsilateral ataxia and falls toward the affected side. The involvement of other parts of the medulla leads to dysphagia, hoarseness, and ipsilateral Horner syndrome. On the contralateral side, the patient may present with spinothalamic sensory loss and mild hemiparesis.[17][18]