Continuing Education Activity
Tubulointerstitial nephritis (TIN) describes a group of immune-mediated inflammatory diseases that involve the interstitium and renal tubules but spare the glomeruli. Inflammation of the kidney consists of the collection of inflammatory cells, fluid, and extracellular matrix surrounding the interstitium, along with the infiltration of tubular cells by inflammatory cells that define both tubules and interstitium pathology. The most common cause of acute interstitial nephritis (70% to 75%) is drugs, also called allergic interstitial nephritis, which is reviewed in a companion activity, "Allergic and Drug-induced Interstitial Nephritis." This activity reviews the remaining 20% to 25% of tubulointerstitial nephritides caused by the following etiologies: idiopathic, genetic, and infectious (viral, bacterial, parasitic, or fungal). In addition, there is an association with various systemic inflammatory conditions such as inflammatory bowel disease, sarcoidosis, systemic lupus erythematosus, Sjögren disease, immunoglobulin G4–associated autoimmune disease, and tubulointerstitial nephritis and uveitis syndrome. This activity highlights the role of interprofessional team members in managing patients with tubulointerstitial nephritis for best patient outcomes.
Objectives:
Identify the clinical signs and symptoms associated with tubulointerstitial nephritis (TIN) to facilitate early diagnosis.
Conduct thorough diagnostic assessments, including laboratory tests, renal imaging, and histological examination, to confirm the diagnosis accurately.
Assess the progress of TIN treatment, monitor renal function, and identify potential complications or relapses.
Communicate the diagnosis, treatment options, and prognosis of TIN to patients and their families, ensuring informed decision-making.
Introduction
Tubulointerstitial nephritis (TIN), also called interstitial nephritis, was first described in 1898 by a pathologist who noted that post-mortem biopsies of patients with streptococcal infection commonly showed nonsuppurative inflammatory lesions in the kidney interstitium. In this group of disorders, nephritis consists of inflammatory cells and fluid within the extracellular matrix of the renal interstitium, along with infiltration of renal tubular cells by macrophages, lymphocytes, plasma cells, and sometimes eosinophils. The glomeruli are usually spared unless chronic TIN develops.[1][2] TIN can be classified as acute or chronic based on the underlying etiology, duration, and histology.[3] By far, the most common cause of TIN is drug-induced, also called allergic, acute interstitial nephritis, which makes up 70% to 75% of TIN cases and is described in a separate StatPearls review. This review describes the etiologies and treatments of non-drug-related TIN, which comprise 20% to 25% of cases.
There are multiple causative factors of non-drug-related TIN, such as idiopathic, genetic, immune-mediated, and infectious (viral, bacterial, parasitic, or fungal). Associated systemic conditions include inflammatory bowel disease, sarcoidosis; systemic lupus erythematosus (SLE); Sjögren disease; immunoglobulin G4-associated autoimmune disease; anti-tubular basement membrane disease; and tubulointerstitial nephritis and uveitis syndrome (TINU).[4][5][6][7][8]
A delay in diagnosis due to TIN's nonspecific signs and symptoms is frequently seen, and such delays can have serious consequences.[2][9] Many attempts have been made to establish an efficient, reliable diagnostic protocol to quickly exclude other differentials through diagnostic tests, clinical history, and disease presentations. However, diagnosing TIN often remains challenging. This review summarizes the diagnosis and management of the various forms of tubulointerstitial nephritis.
Autosomal dominant tubulointerstitial kidney disease is described in StatPearls companion reference on "Autosomal Dominant Tubulointerstitial Kidney Disease."[10]
Etiology
Infection
Infections are the second-most common cause of TIN after drugs in developed countries; they are the leading cause in undeveloped countries.[11] The cause of infections can be broken down into the following categories:
- bacteria: Escherichia coli, Campylobacter, Salmonella, Streptococci, Mycoplasma, Yersinia
- viruses: HIV, cytomegalovirus, Epstein-Barr, polyoma, herpes simplex, COVID-19
- fungi: Histoplasma, Coccidioides
- parasites: Toxoplasma, Leishmania, Giardia
Patients with infectious causes present with symptoms resulting from the underlying infection, and AKI is rarely the presenting symptom. Treatment of the offending agent is required before steroid treatment for TIN.
Autoimmune Disease
Autoimmune diseases are an important cause of TIN, including systemic lupus erythematosus (SLE), Sjogren syndrome, sarcoidosis, inflammatory bowel disease, and the recently discovered IgG4-related disease.
Lupus nephritis has traditionally been associated with glomerular injury; however, recent evidence shows that interstitial fibrosis and tubular atrophy may be more important factors in determining response to treatment and prognosis of renal recovery. Lupus nephritis affects up to 40% of adults and 80% of children affected by SLE. Despite treatment advances, 10% of patients will progress to ESRD and need renal replacement therapy. SLE is characterized by autoantibodies triggering the deposition of immune complexes in the glomeruli, complement system activation, and leukocyte recruitment. The characteristic anti-double-stranded DNA antibodies promote fibronectin and collagen production, ultimately leading to extracellular matrix fibrosis. There is evidence that interstitial fibrosis and tubular atrophy may be better predictors of renal outcome than glomerular injury and may also be a target for reversible fibrosis progression.[12][13]
Sjogren syndrome less commonly involves the kidney (fewer than 10% of cases). When there is kidney involvement, TIN is the most common pathology observed, in which interstitial plasma cell infiltration is a key feature. Clinical presentations often involve distal tubular acidosis, nephrogenic diabetes insipidus, Gitelman syndrome, or Fanconi syndrome.[14][15]
In Western countries, granulomatous TIN is usually associated with drugs or sarcoid, whereas in countries with a high infectious burden (such as India), tuberculosis is the most common pathogen. Granulomatous TIN due to sarcoid often shows well-demarcated granulomas containing renal epithelioid histiocytes and multinucleated giant cells with a peripheral cuff of lymphocytes and plasma cells. Caseating granulomas are associated with tuberculosis or fungal infection. Sarcoidosis often causes altered vitamin D and calcium metabolism, which can be important diagnostic clues.[14][16]
Inflammatory bowel disease (IBD) is associated with renal dysfunction in up to 23% of cases. One review showed TIN as the second-most common pathology observed when renal biopsy was conducted for AKI (after IgA nephropathy). Some cases were thought to be due to aminosalicylate exposure, but a significant number were not thought to be drug-related.[17] Another study showed that the presence of tubular proteinuria (likely secondary to TIN) correlated with IBD activity but not with drug administration. TNF-alpha is suggested as an inflammatory mediator causing both active IBD and TIN.[18]
IgG4-related systemic disease causes direct tubulointerstitial damage by direct toxicity of protein overload, possibly through lysosome-induced cellular injury. The severity and chronicity of the disease correlate with the amount of proteinuria and may be linked to alternative complement pathway activation. IgG-positive plasma cells will be visualized in inflammatory regions on kidney biopsy, and this disease responds well to steroids.[19] TIN is the most common pathology seen with IgG4 disease; however, IgG4 can also form deposits indistinguishable from malignancy on imaging. This can lead to unnecessary nephrectomy, so a high index of suspicion must be maintained to consider this rare disease on the possible differential of renal masses.[20]
Transplant TIN
Tubulointerstitial nephritis is the third leading cause of renal transplant dysfunction and is often due to underlying infection. Common infections causing TIN in transplanted kidneys include bacterial pyelonephritis and the BK virus infection. In contrast, adenovirus, JC virus, and cytomegalovirus are less frequent but can also lead to significant allograft dysfunctions.[3] BK virus is found quiescently in most adults. Because of immunosuppression, the usually latent virus can become active in patients with transplants, causing inflammation. Ongoing TIN can cause permanent fibrosis and allograft dysfunction and decrease the life of the transplanted kidney.
Histologically, the interstitium is infiltrated by inflammatory cells. Immunohistology and electron microscopy may show viral inclusions in renal tubular epithelial cells. TIN must be differentiated from allograft rejection.[21]
Anti-TBM disease is another rare form of glomerular-sparing inflammatory nephritis caused by anti-tubular basement membrane (anti-TBM) antibodies directed against the tubular interstitial nephritis antigen. This antigen is an extracellular basement membrane protein located in the proximal renal tubules and regulates tubular growth.[4][22] The usual presenting symptoms are polyuria and polydipsia. On laboratory analysis, microscopic hematuria and mild proteinuria are seen, along with anti-TBM serum antibodies.[22] Drugs can also induce the formation of anti-TBM antibodies.[4][22] Anti-TBM disease is seen in all age groups.[22]
In addition to tubulitis, histopathology shows linear IgG and C3 deposits in the proximal renal tubules.[22][23][24] The glomeruli and distal tubules are typically spared.[23]
Tubulointerstitial nephritis and uveitis syndrome (TINU) is a rare disease associated with the Epstein-Barr virus and bacteria like Leptospira, Mycoplasma, and Yersinia.[25][26] It usually presents initially with renal failure from TIN followed by bilateral uveitis, although the timing can be variable.[27] Ocular symptoms of uveitis include photophobia, eye pain, and redness, but up to 50% of patients may not experience ocular symptoms. TINU is more common in females and is most often found in children and teens younger than twenty (in whom TINU accounts for up to 33% of all uveitis cases).[27] The exact pathophysiological mechanism of TINU is unclear, but autoimmune, genetic predisposition, iatrogenic, and infectious factors appear to be involved.[28][29]
The finding of tubulointerstitial nephritis on renal biopsy, together with the clinical finding of bilateral uveitis, establishes the diagnosis. Alternatively, TINU can be diagnosed by uveitis associated with all 3 of the following criteria:
- Elevated serum creatinine or lower GFR
- Abnormal urinalysis (hematuria, proteinuria, eosinophiluria, casts, or pyuria without infection)
- Systemic illness (eg, fatigue, fever, weight loss, anemia, elevated liver enzymes) lasting 2 weeks or more [30]
Epidemiology
The global prevalence of acute AIN due to any cause is 1% to 3% among all renal biopsies. The overall prevalence of acute AIN increased to 15%-27% when the analysis was restricted to only AKI cases.[31][32][33][34][35] Developed countries have a much higher proportion of drug-induced AIN than infection or autoimmune, while in developing countries, infectious causes are the most prevalent. Older patients are much more likely to have drug-induced AIN than systemic or autoimmune disease due both to the increased use of drugs and less active immune systems in this population. Each of the categories above has different demographic predominances. For example, TINU is a disease primarily of children and teenagers younger than 20, while anti-TBM disease is found in all age groups. Women tend to have much higher rates of SLE and Sjogren syndrome, while the distribution of sarcoidosis is about equal between the sexes.
Pathophysiology
High metabolic demand and relatively low blood supply make the tubulointerstitium susceptible to injury.[36] TIN involves inflammation and edema of the renal tubules and interstitium, compromising the blood supply and ultimately causing a decrease in the glomerular filtration rate (GFR). The glomeruli are relatively spared and involved only in the late stages.
In TIN, production and activation of cytokines, such as tissue necrosis factors α and ß (TNF-α and -ß), promote the accumulation of collagen in the extracellular matrix and basement membrane, as well as inhibit collagenase and metalloproteinase enzymes resulting in fibrosis.[37][38][39] Some studies suggest that the production of reactive oxygen species during oxidative stress induces mitochondrial injury; damaged mitochondria then release proapoptotic factors like cytochrome c.[36]
The pathophysiology of infectious TIN is dependent upon the microbial agent. Renal histology shows interstitial edema with significant neutrophilic presence and typical lymphocytic infiltrates. Immunofluorescence (IF) is generally negative for complement or specific Ig deposits.[40] Infection-related TIN is often responsive to steroids, similar to other forms, but the infection must be adequately treated before starting immunosuppression.[40]
The pathogenesis of other systemic diseases, such as sarcoidosis, inflammatory bowel disease, SLE, granulomatosis with polyangiitis, and Sjögren syndrome, is complex. Multiple factors, such as susceptibility to autoimmunity, genetic predisposition, nutritional insufficiency, and predisposed infectious agents, are thought to be involved.[3]
Chronic TIN refers to long-standing and progressive cases characterized by irreversible deterioration of renal function due to tubular atrophy and fibrosis. Tubular atrophy leads to a decrease in the number of active nephrons. This overwhelms the functional capacity of the remaining nephrons by hyperfiltration, eventually leading to chronic kidney disease (CKD). Chronic TIN can be caused by the same processes that result in acute TIN, especially if there is an underlying systemic autoimmune disorder.[41] It has been associated with Alport syndrome, amyloid, cystinosis, transplant nephropathy, chronic drug use, kidney stones disease, lead and mercury toxicity, leukemia, multiple myeloma, and urinary obstruction.
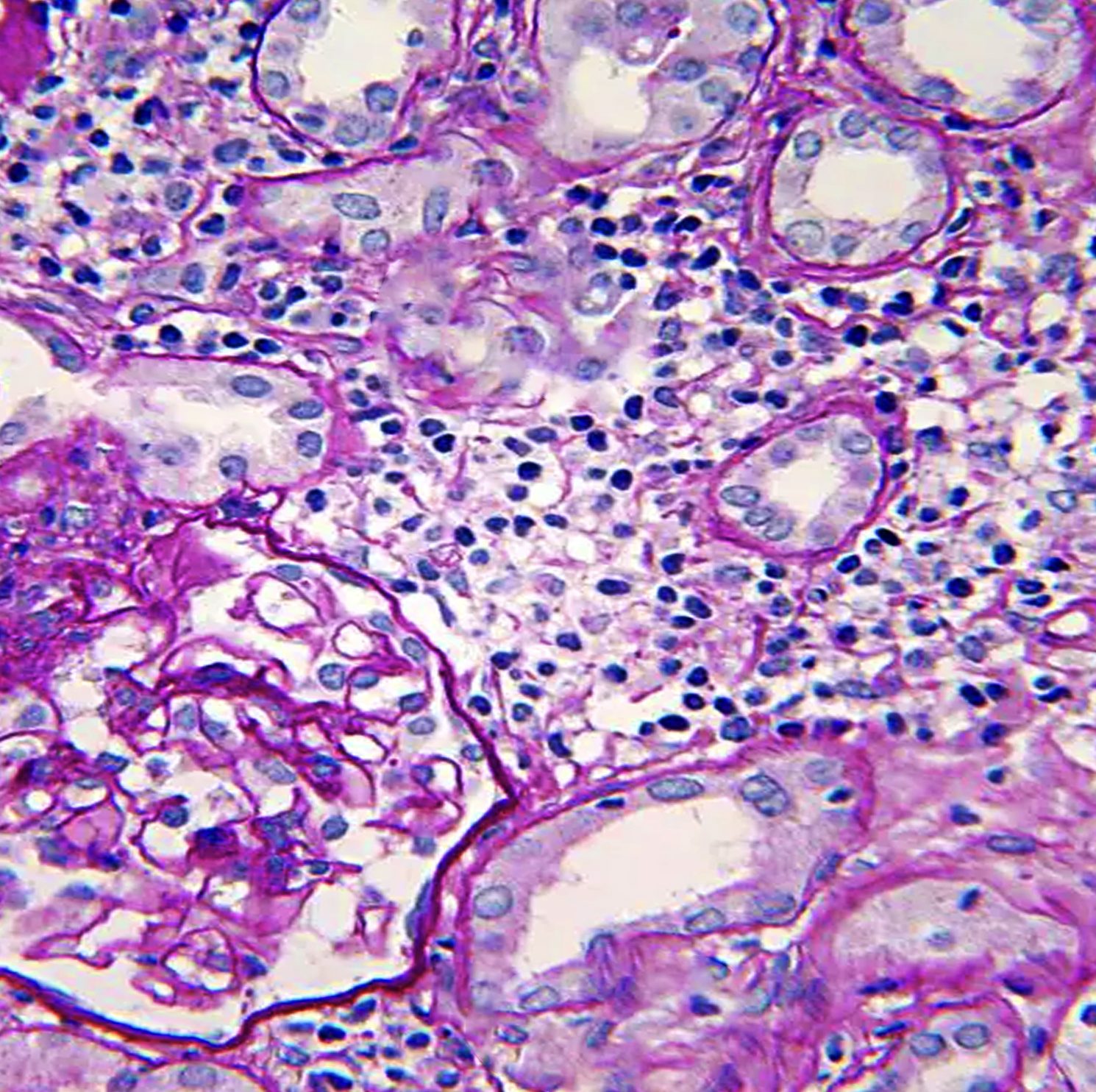
Histopathology
Histopathologic light microscopy in cases of TIN reveals interstitial inflammatory cell infiltration and edema, regardless of etiology, usually with a predominant lymphocytic infiltration. The presence of dominant neutrophils and plasma cells suggests a bacterial infection.[3][42] In contrast, acute tubular necrosis will show primary tubular damage and casts with less interstitial involvement.
Interstitial inflammation is either focal or diffuse. In addition, features of tubulitis are present, including tubular degenerative changes, irregular luminal contours and ectasia, cytoplasmic vacuolization, prominent nucleoli, apoptotic cells, loss of brush border, and tubular atrophy.[43][44] Granuloma formation with multinucleated giant cells and well-demarcated granulomas suggest sarcoidosis while caseating or necrotic granulomas are suspicious for tuberculosis infection.[14][16] In autoimmune diseases such as SLE and Sjogrens, immunofluorescence often shows granular deposits of immune complexes in the kidney (and other organs). This is rarely seen in other forms of TIN. IgG4-related TIN may show C3 deposition in the renal interstitium accompanied by low serum complement levels.[3]
In cases of BK-induced TIN in transplanted kidneys, monocytic rather than lymphocytic infiltration predominates, and basophilic viral inclusions are often seen in the nuclei of renal tubular interstitial cells. Viral inclusions may not be apparent in early infection, but staining for Simian Virus 40 (SV40) staining will be present. Medullary changes are often more visible than cortical changes, which is noteworthy as renal biopsies often contain only cortical tissue.
History and Physical
A challenging aspect of TIN is the nonspecific and highly variable clinical presentation that delays the diagnosis and treatment. History of onset and duration, patient age, and drug intake are of utmost importance while assessing for TIN. The traditional triad of fever, rash, and eosinophilia occurs variably and rarely together. Arthralgias are common but nonspecific.
- Autoimmune and infectious diseases present with extra-renal symptoms dependent on the underlying pathology.
- Early presentations of TIN may show normal or increased urination due to defects in renal tubular reabsorption, while undetected or worsening TIN may develop into oliguria if the TIN progresses.[45]
- Flank pain and a sense of abdominal fullness or fullness radiating to the groin may reflect distention of the renal capsule by edema caused by TIN.[45]
- Detectable features of nephrotic syndrome, like facial puffiness and pedal edema, may also be seen.[46]
- Ocular symptoms are usually associated with TINU in children and teenagers younger than 20 and Sjogrens in patients older than 20. Of note, uveitis does not always present with ocular symptoms, and a detailed ophthalmologic exam is required.[3]
Evaluation
The diagnosis of tubulointerstitial nephritis is often delayed due to its nonspecific symptoms. Differentiating acute and chronic TIN is challenging. It is essential to separate TIN from other causes of renal diseases, such as glomerulonephritis and acute tubular necrosis, because the treatment and prognosis are quite different. One should always have a high suspicion of TIN in any patient with renal insufficiency. Clinical assessment, laboratory findings, and imaging tests are the approaches currently used to make the diagnosis.
Blood Tests
The most significant initial manifestation of TIN is renal failure, as evidenced by increased blood urea nitrogen and serum creatinine. This is often an incidental finding in asymptomatic patients. The diagnosis of TIN should always be considered when patients develop an unexplained rise in serum creatinine levels.[43] A BUN/creatinine ratio of 12 or less is highly suggestive of TIN.[47] Levels of complement factors, anti-neutrophilic cytoplasmic autoantibody, immunoglobulin subtypes, and anti-streptolysin O antibody should be tested.
Electrolyte and acid-base disturbances include hyperkalemic, hyperchloremic metabolic acidosis that is out of proportion to the degree of kidney failure, raising the possibility of associated TIN.[48][49] Low serum phosphorus and uric acid levels also suggest a Fanconi-type syndrome, which also points to tubular injury. Distal tubular injury can also cause renal tubular acidosis. Decreased erythropoietin synthesis due to tubular cell injury and erythropoietin resistance often leads to anemia. Elevated serum erythrocyte sedimentation rate (ESR) or the C-reactive protein level suggests an inflammatory process but is nonspecific.
Imaging Tests
Renal ultrasonography and CT scans are often used as imaging studies to support the diagnosis of TIN, primarily by excluding urolithiasis, cysts, masses, obstruction, and hydronephrosis. These tests typically reveal bilateral normal or mildly enlarged kidneys and diffusely increased cortical hyperechogenicity.[43][48]
Gallium scintigraphy has been used to show interstitial enhancement but has limited sensitivity and specificity. An indication for a gallium scan is a contraindication to renal biopsy or patient refusal.[44] PET scans can also be used in the diagnosis of TIN, but like gallium scanning, they have limited sensitivity and specificity.
Urinalysis and Microscopy
Microscopic urinalysis is one of the most commonly used tests to investigate renal disorders and can often provide helpful clues to diagnosing TIN. Proteinuria (usually less than 1gm/day), pyuria without evidence of bacterial infection, WBC casts, and microscopic hematuria are common findings.[3][44]
Urinary Biomarkers
The development of clinically useful urinary biomarkers to identify and monitor TIN activity is a potential area for diagnosing and following TIN. Biomarkers such as monocyte chemotactic peptide-1 (MCP-1), alpha1-microglobulin (A1M), matrix metalloproteinase-2 (MMP-2), and particularly beta 2- microglobulin (B2M) and CXCL9 appear quite promising.[3][50] These low molecular weight proteins suggest tubular injury and inflammatory interstitial pathology when present in the urine.
Some early findings include:
- Azotemia with an elevated urinary beta2-microglobulin had a 100% positive predictive value in identifying patients likely to have tubulointerstitial cystitis and uveitis syndrome (TINU.)[51]
- B2M has higher sensitivity and specificity than A1M for tubulointerstitial nephritis.[52]
- MCP-1 is usually observed in drug-induced TIN.[3]
- Urinary levels of CXCL9, a chemokine involved in the pathophysiology of TIN, are more than 7 times higher in TIN patients than in controls.[53][54]
Renal Biopsy
Histopathological examination after a renal biopsy is the definitive gold standard for reliably diagnosing TIN. If there is no clinical improvement even after the withdrawal of all potential offending drugs for 5 to 7 days, a renal biopsy should be performed, provided no contraindications.[55] Contraindications for a renal biopsy include an uncorrected bleeding diathesis, inability to stop anticoagulation due to comorbidities, inability of the patient to adequately cooperate for the procedure, end-stage renal disease with atrophic kidneys, uncontrolled hypertension, active infection (UTI, pyelonephritis, sepsis), hemodynamic instability, and patient refusal. A solitary kidney is a relative contraindication and should only be considered when necessary in selected cases.[55]
Treatment / Management
It is essential to have high clinical suspicion to eliminate causative agents of tubulointerstitial nephritis and treat any associated systemic disorders. Corticosteroids have been the mainstay treatment against most kinds of TIN, but clinical trials do not consistently support the benefits of corticosteroid therapy.[56][57][58] Given the relative safety of limited corticosteroid use, most clinicians will prescribe a defined course if there is no contraindication. In infection-related TIN, the systemic infection is treated prior to corticosteroid administration.
TINU is usually treated with systemic corticosteroids, but the uveitis may also be treated with topical steroids and cycloplegic agents. If systemic steroids are not tolerated, immunomodulatory agents— such as methotrexate, azathioprine, and mycophenolate mofetil—have been used.[3][23] Therapeutic plasma exchange has been proposed as a possible treatment to remove circulating anti-TBM antibodies, but there is no data on whether this therapy is beneficial.[23][24]
Transplant patients with higher levels of immunosuppression are at higher risk of TIN caused by viral and bacterial infections. Viral- or bacteria-related TIN in renal transplant is managed by reducing immunosuppressants, and the antiviral cidofovir is often indicated.[59]
Differential Diagnosis
Clinical presentations and laboratory results of TIN are not specific but overlap with most kidney diseases that cause AKI and renal insufficiency. When assessing suspected TIN in patients with renal insufficiency, the following problems should be considered:
Acute Tubular Necrosis: ATN is the most common cause of acute renal failure characterized by tubular cell necrosis for various reasons, such as ischemia, nephrotoxins such as aminoglycosides, heavy metals, urate, radiocontrast dye, or other toxic agents. In addition, manifestations like oliguria, metabolic acidosis, elevated BUN and creatinine, and electrolyte imbalances are similar to TIN. Muddy brown or granular casts are more likely with ATN.[60]
Atheroembolism: Atheroembolism (cholesterol crystal emboli) should be considered in patients with a predominance of urinary WBC and RBC casts. Atheroemboli may also present with skin rashes, eosinophiluria, and eosinophilia. Skin changes usually include livedo reticularis and digital infarcts rather than the diffuse maculopapular rash of TIN. A history of endovascular diseases, older age, and obesity point towards atheroemboli.[61]
Glomerulonephritis: A wide range of glomerulopathies ultimately can lead to renal impairment and mimic TIN to some degree. Some presentations of glomerulonephritis are similar to TIN, such as proteinuria and oliguria. WBC casts and dysmorphic RBCs are suggestive of glomerulonephritis rather than TIN.[62][63]
Obstructive Uropathy: Urinary tract obstruction is a postrenal cause of acute renal failure. It is usually attributed to renal stones, tumors, and strictures and may cause anuria. In such patients, imaging helps to distinguish urinary obstruction from other causes of AKI.[64] Obstruction can also cause urinary infections and must be treated to treat the infection effectively.[64][65]
Vascular Injury: Various cardiovascular insults, such as renal artery stenosis, cardiac failure, vasculitis, reduced blood flow due to afferent arteriolar constriction in NSAID users, and reduced efferent arteriolar tone by ACE inhibitors, are common causes of AKI that clinically simulates TIN. The rash in vasculitis is typically purpura, while an allergic-type maculopapular rash would be more likely with drug-induced TIN.
Prognosis
The prognosis depends on the cause of TIN, the timing of therapy, baseline renal function, prior offending agents, and exposure time to the underlying trigger. Chronicity, such as extensive fibrosis or tubular atrophy, portends worse outcomes. Early identification and removal of the cause improve renal outcomes.
Infectious causes of TIN are usually self-limited and respond well to antimicrobial treatment. Autoimmune-related TIN often relapses depending on the activity of the underlying disease, and renal function should continue to be followed. TINU, in particular, requires close ongoing follow-up with ophthalmology due to its recurrent nature and young cohort. Patients with kidney transplants who experience TIN due to viral causes should have closely monitored immunosuppressant and viral load levels, as each TIN episode can potentially worsen allograft function and precipitate a rejection episode.
Complications
Older patients are more vulnerable to complications. Renal insufficiency is a common manifestation that ultimately progresses to ESRD due to fibrosis of the interstitial and degeneration of tubular epithelial cells. In addition to renal insufficiency, inflammation or infection of proximal tubular cells can result in either decreased synthesis or hyporesponsiveness to erythropoietin by injured cells, leading to reduced RBC production by bone marrow and complications of anemia.[44] TIN also increases angiotensin II activity, which leads to arterial hypertension due to sodium and fluid retention, vasoconstriction, and increased oxidative stress.[66][67] Increased angiotensin concentration changes the hemodynamic status and oxidative stress, causing vasoconstriction.[68][69]
Deterrence and Patient Education
Patients should be educated as to the underlying cause of TIN and ways to manage or avoid future recurrence. All diagnosis testing and resulting information should be thoroughly discussed with the patient, and treatment options with risks and benefits. Information provided to patients about the disease and their course of treatment should be understandable.
Pearls and Other Issues
A renal biopsy is the gold standard diagnostic test for TIN, but it cannot always identify the underlying etiology. Nephrotic syndrome (3 grams or more of urinary protein excretion per day) is rare with tubulointerstitial nephritis except with NSAID-related drug-induced TIN.
While drug-induced TIN is the most commonly seen, other etiologies may be involved. Selected patients may benefit from further testing to help identify any underlying disorders. Such testing may include any of the following:
- Chest x-ray or chest CT to screen for tuberculosis and sarcoidosis.
- Serum antibody tests for ANCA, SLE, tuberculosis, syphilis, streptococcus, Epstein-Barr virus, and HIV.
- Serum protein electrophoresis.
- Serum rheumatoid factor, antinuclear antibodies, angiotensin-converting enzyme to screen for autoimmune disease. Serum calcium, serum vitamin D, and 24-hour urinary calcium are useful to screen for Sjogren syndrome and sarcoidosis.
- Serum C3 and C4 will be low in cases of SLE and IgG4.
- Slit lamp examination for uveitis or eye pain associated with tubulointerstitial nephritis and possible TINU.
Enhancing Healthcare Team Outcomes
TIN has a wide range of etiologies that require clinicians from various specializations, such as nephrologists, urologists, infectious disease experts, ophthalmologists in cases of TINU, dermatologists, pathologists, and rheumatologists.[30] These disciplines coordinating their efforts, maintaining meticulous patient records, and utilizing open communications will bring about the best possible patient outcomes.
Diagnostic approaches can be made more effective with the help of experienced histopathologists, radiologists, lab technicians, and microbiologists. Skin rashes can be evaluated and managed with the help of dermatologists. Nursing staff is crucial to ensure proper monitoring of patients, and pharmacists provide drug education and review adverse effects. Radiologists have their role in kidney biopsies, and urologists perform renal transplantation for end-stage kidney failure. In this way, the comprehensive interprofessional healthcare team achieves better results with fewer adverse outcomes.