Continuing Education Activity
One of the most common causes of emergency room visits in the United States is status asthmaticus, an acute, emergent bronchial asthma episode poorly responsive to standard therapeutic measures. This condition can rapidly escalate into acute ventilatory failure and be potentially fatal if not recognized and treated promptly. Timely diagnosis and management are vital to improving outcomes for patients with status asthmaticus.
This activity for healthcare workers is designed to enhance learners' competence in evaluating and managing status asthmaticus. This course emphasizes the need for interprofessional team coordination when caring for patients with this condition.
Objectives:
Identify early signs and symptoms of status asthmaticus, distinguishing them from routine exacerbations.
Implement evidence-based management strategies promptly, tailored to the severity of status asthmaticus.
Select appropriate pharmacological agents and delivery methods based on the patient's individual characteristics and response to treatment.
Collaborate effectively with the interprofessional team in developing short- and long-term care strategies for patients with status asthmaticus.
Introduction
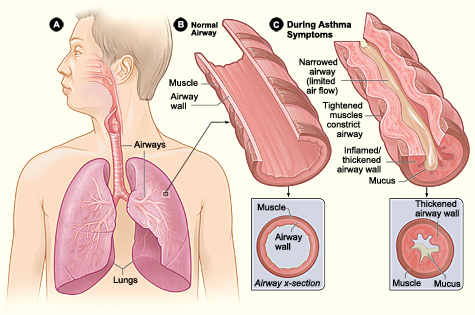
Asthma is a chronic inflammatory airway disease marked by recurrent wheezing, dyspnea, chest pain, and coughing (see Image. Asthma Pathology). The recommended treatments for acute attacks with mild-to-moderate symptoms include β2-agonists and steroids. However, status asthmaticus arises when patients fail to respond to standard asthma treatments.
Status asthmaticus is a medical emergency, an extreme form of asthma exacerbation characterized by hypoxemia, hypercarbia, and secondary respiratory failure. All patients with bronchial asthma are at risk of developing this condition, which may progress and become poorly responsive to standard therapeutic measures. If not recognized and managed appropriately, status asthmaticus can lead to acute ventilatory failure and even mortality.
Status asthmaticus remains one of the most common causes of emergency department visits despite pharmacotherapeutic advances. No single clinical or diagnostic index has been known to predict the clinical outcomes of this condition. A multi-pronged approach, combining clinical evaluation, appropriate diagnostic tests, and rapid symptom relief, can improve outcomes for patients with this condition.
The Normal Lung
Embryologically, the respiratory system is a ventral foregut outgrowth. The trachea develops at the midline and gives rise to the lung buds. The right lung bud divides into 3 main bronchi, while the left divides into 2. The right main stem bronchus is more vertical than the left, thus more vulnerable to foreign body aspiration. Both right and left main bronchi branch into progressively smaller airways, forming the bronchioles, lobules, terminal bronchioles, acini, respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli. Pulmonary and bronchial arteries follow airway and lung parenchymal branching. Several terminal bronchioles form a pulmonary lobule.
Pseudostratified columnar ciliated epithelium covers the laryngeal, tracheal, and bronchial surfaces. Cartilaginous tissue also supports these airways. Neuroendocrine cells abound in the bronchial mucosa, secreting serotonin, calcitonin, and bombesin. Submucosal mucus glands are also found in the tracheal and bronchial walls.
Cartilage and submucosal mucus glands disappear at the bronchiolar level. Terminal bronchioles have a diameter of less than 2 mm. Acini are spherical structures distal to the terminal bronchioles with a 7 mm diameter and comprised of respiratory bronchioles. The alveolar ducts arise from the respiratory bronchioles and branch into the alveolar sacs. Alveoli can arise from respiratory bronchioles and alveolar ducts and sacs.
Alveoli are the lungs' gas exchange units. The blood-air barrier comprises the capillary epithelium, basement membrane, interstitial tissue, alveolar epithelium, and alveolar macrophages. Type I pneumocytes are the flat cells constituting 95% of the alveolar epithelium. Type II pneumocytes are rounded cells occurring more sparsely in the alveolar space. Type II pneumocytes secrete pulmonary surfactant and repair the alveolar epithelium when type I pneumocytes are damaged. Pores of Kohn on the alveolar walls are potential sites for microbial and exudate spread between alveoli.
Etiology
Asthma is a chronic inflammatory disorder of the airways that produces recurrent episodes of difficulty breathing. This condition is also associated with increased hyperreactivity to certain stimuli.[1]
The progression time course and airway obstruction severity follow 2 distinct patterns.
- Slow-onset asthma exacerbation: characterized by a slow, subacute worsening of the peak expiratory flow rate (PEFR) over days. Patient-related predisposing factors, including inadequate inhaler regimen, suboptimal compliance, and psychological stressors, are often present.
- Sudden-onset asthma exacerbation: presents with severe deterioration within hours. This condition is often precipitated by sudden massive exposure to external triggers like allergens, food particles, and sulfites.[2]
About 80% to 85% of asthma-related fatalities consist of patients who experience slow-onset asthma exacerbations, reflecting inadequate disease control over time. Patients with slow-onset asthma exacerbations have extensive airway inflammation and mucus plugging. In contrast, patients who experience sudden-onset exacerbation present mostly with clear airways.[3]
Epidemiology
Centers for Disease Control and Prevention (CDC) data reveal that the overall prevalence of asthma in the United States has decreased from 7.9% in 2017 to 7.7% in 2021. The prevalence of this condition is higher in adults than the pediatric population (8.0% vs 6.5%).
Multiple observational studies have reported a higher incidence in women, African Americans, and people with asthma onset after 17 years of age.[4][5] Status asthmaticus is more prevalent in individuals with lower socioeconomic status due to limited specialist care access.[6] Those who live alone are disproportionately affected. According to the CDC, 10.4% of those with asthma are below the 100% poverty threshold. Blacks and American Indians (Alaska natives) have the highest prevalence rates at 10.9% and 12.3%, respectively.
An estimated 3% to 16% of hospitalized adult asthmatic patients develop respiratory failure requiring ventilatory support, although the statistics might be lower in children. Afessa et al reported a mortality of around 10% in patients with status asthmaticus admitted to the intensive care unit (ICU).[7]
Increasing tidal-volume-ventilation strategy standardization, avoiding prolonged neuromuscular blockade, and assist-control mode ventilation have reduced mortality over the past decade. A retrospective review revealed that 61.2% of 280 patients treated for asthma in San Antonio, Texas, required intubation and mechanical ventilation. The mortality rate was about 0.35%.[8] A retrospective study by the Collaborative Pediatric Critical Care Research Network found a mechanical ventilation rate of 11.5% and an overall mortality rate of approximately 2% in over 13,000 children treated for asthma.[9]
Pathophysiology
Asthma arises from chronic inflammation in the lower airways. Inflammation is multi-factorial, including genetic, environmental, and the patient's microbiome.[10] Premature airway closure during exhalation increases functional residual capacity and causes air trapping. The heterogeneous distribution of air trapping results in ventilation-perfusion mismatch and hypoxemia, triggering anaerobic metabolism and lactic acidosis. Respiratory alkalosis initially offsets the acidosis until respiratory fatigue ensues.
Physiologically, acute asthma is divided into 2 phases. An early bronchospastic phase is observed minutes after allergen exposure, with mast cell degranulation and the release of inflammatory mediators like histamine, prostaglandin D2, and leukotriene C4. A later inflammatory phase causes airway swelling and edema due to the eosinophilic release of eosinophilic cationic proteins and major basic proteins.[11] Specifically, type 2 inflammation appears to be a key risk factor in acute asthma exacerbation. This process is characterized by the release of interleukins 5, 4, and 13 produced by T-helper type 2 cells and type 2 lymphoid cells. Additionally, non-type 2 inflammation involving neutrophils likely plays a significant role in asthma exacerbations.[12]
Histopathology
Histopathological examination primarily shows evidence of airway inflammation, smooth muscle contraction, and airway hyperresponsiveness. Mast cell, T lymphocyte, and epithelial cell interactions result in a circulatory surge of inflammatory cells and cytokines. Histamines, leukotrienes, and platelet-activating factors are found in increased concentrations locally and systemically. Lymphocytic and eosinophilic submucosal infiltrates in tracheal and bronchial biopsy specimens are reportedly associated with poorer outcomes in adult patients with asthma.[13]
Cilia destruction and epithelial denudation render nerve endings irritable, resulting in hyperreactivity. Inflammation also causes hypertrophy and hyperfunctioning of goblet cells and mucous glands, resulting in mucus plugging. A dysregulated parasympathetic drive contributes to cellular-level pathology, mediated by the pulmonary vagal branches and small bronchi's parasympathetic ganglia. Postganglionic acetylcholine muscarinic receptor stimulation causes bronchoconstriction and hypersecretion. Inhibitory M2 receptors are often dysfunctional in individuals with atopy, sustained exposure to allergens, viral infection, and chronic inflammation.
History and Physical
Patients with asthma exacerbation most often present with a chief complaint of shortness of breath. Individuals may also report increasing inhaler or nebulizer use within the past few hours or days. Fever, upper respiratory infection symptoms, cough, and chest pain may be present. Pertinent information that must be elicited on history includes illicit substance use, comorbidities, current medications, and known allergic triggers.
Several risk factors predispose patients to developing status asthmaticus.[14][15] A history of near-fatal asthma requiring endotracheal intubation is the greatest single predictor of death from bronchial asthma. Similarly, poor patient perception of dyspnea and hypercapnia may also lead to status asthmaticus. A blunted hypoxic ventilatory response results from longstanding pulmonary disease or psychiatric illness.[16][17] Recurrent hospitalizations despite chronic oral steroid use, late presentation since symptom onset, altered mental status, and sleep deprivation during the exacerbation episode also increase the risk of status asthmaticus. An existing coronary artery disease risks cardiological adverse events with asthma therapy.
On physical examination, patients are typically tachycardic, tachypneic, and conversationally dyspneic. Wheezing on pulmonary auscultation is often used as an indicator of bronchospasm. However, this finding is not always present in individuals with severe asthma exacerbation or status asthmaticus. Alveolar airflow is significantly impaired during an asthma attack. Therefore, the absence of wheezing should not be used as an indicator of mild disease, particularly if a patient is in extremis.[18][19]
Tachycardia greater than 120 bpm may be an indicator of both disease severity and treatment response to β2-agonists. Grossman et al demonstrated that successful treatment results in a 24-hour heart rate drop from 120 to 105 beats per minute. Sinus tachycardia is the predominant rhythm, although supraventricular and ventricular arrhythmias have also been reported.[20]
Brenner and colleagues demonstrated that patients experiencing asthma exacerbation and assumed an upright rather than a supine position tended to have significantly higher heart and respiratory rates than those who did not. Other findings in these patients were pulsus paradoxus, significantly lower arterial oxygen partial pressure (PaO2), and a lower PEFR. However, status asthmaticus results in progressive clinical and mental status decline that often results in patients resting supine. Thus, patient positioning should not be used to make clinical decisions. After initial treatment, diaphoresis, preference to sit upright, inability to speak complete sentences, and accessory muscle use all suggest progression to status asthmaticus.
A circulatory consequence of status asthmaticus is a large respiratory-phase variation in pleural pressure. The increased inspiratory effort against an obstructed airway augments negative intrathoracic pressure, reducing left ventricular filling and outflow due to the combination of the following:
- Septal deviation to the left due to an enlarged right ventricle
- Increased left ventricular afterload
- Increased right ventricular afterload due to increased pulmonary arterial pressure
Thus, systolic blood pressure tends to fall at the height of inspiration. Pulsus paradoxus exists when the systolic blood pressure falls by at least 10 mm Hg during inspiration. This finding is augmented to more than 12 mm Hg in status asthmaticus, although it may decline in the later stages with increasing fatigability and loss of respiratory drive.[21] Consider preparing for resuscitation in patients at risk of developing status asthmaticus or who may have poor cardiac function.
Evaluation
Patients in cardiorespiratory distress must be hooked to a cardiac and blood pressure monitor and pulse oximeter. Vital signs should be taken at least every 15 minutes while stabilization measures are ongoing. The patient's mental status must also be monitored frequently, as neurological deterioration is a sign of inadequate oxygenation.
Airflow obstruction measurement can be challenging to perform but is best achieved by taking PEFR and forced expiratory volume in 1 second (FEV1) at the bedside.[22] The reduction of both values by at least 50% of the patient's personal best is an indicator of severe airway obstruction. An absolute PEFR value of less than 120 liters per minute and FEV1 of less than 1 liter indicates severe disease. These absolute numbers should prompt arterial blood gas (ABG) assessment.[23]
Laboratory tests and imaging can provide information about treatable asthma triggers, assess disease severity, and rule out other dyspnea etiologies. Initial blood tests may include a complete blood count (CBC), basic metabolic panel (BMP), and ABG. CBC typically shows leukocytosis. Neutrophilia is often associated with bacterial infection. Viral infections may be accompanied by lymphocytosis or mild lymphopenia. The presence of an infection may be confirmed with a nasopharyngeal swab, sputum test, or blood culture.
A metabolic panel can help assess electrolyte balance and kidney function. Dyspnea-related insensible losses and, likely, poor intake may affect these parameters. Cardiac enzymes and electrocardiography can help evaluate cardiac function and rule out acute cardiac pathology. Pulmonary obstruction may manifest on the electrocardiogram with transient and reversible signs of right heart strain, including peaked P waves or right axis deviation.
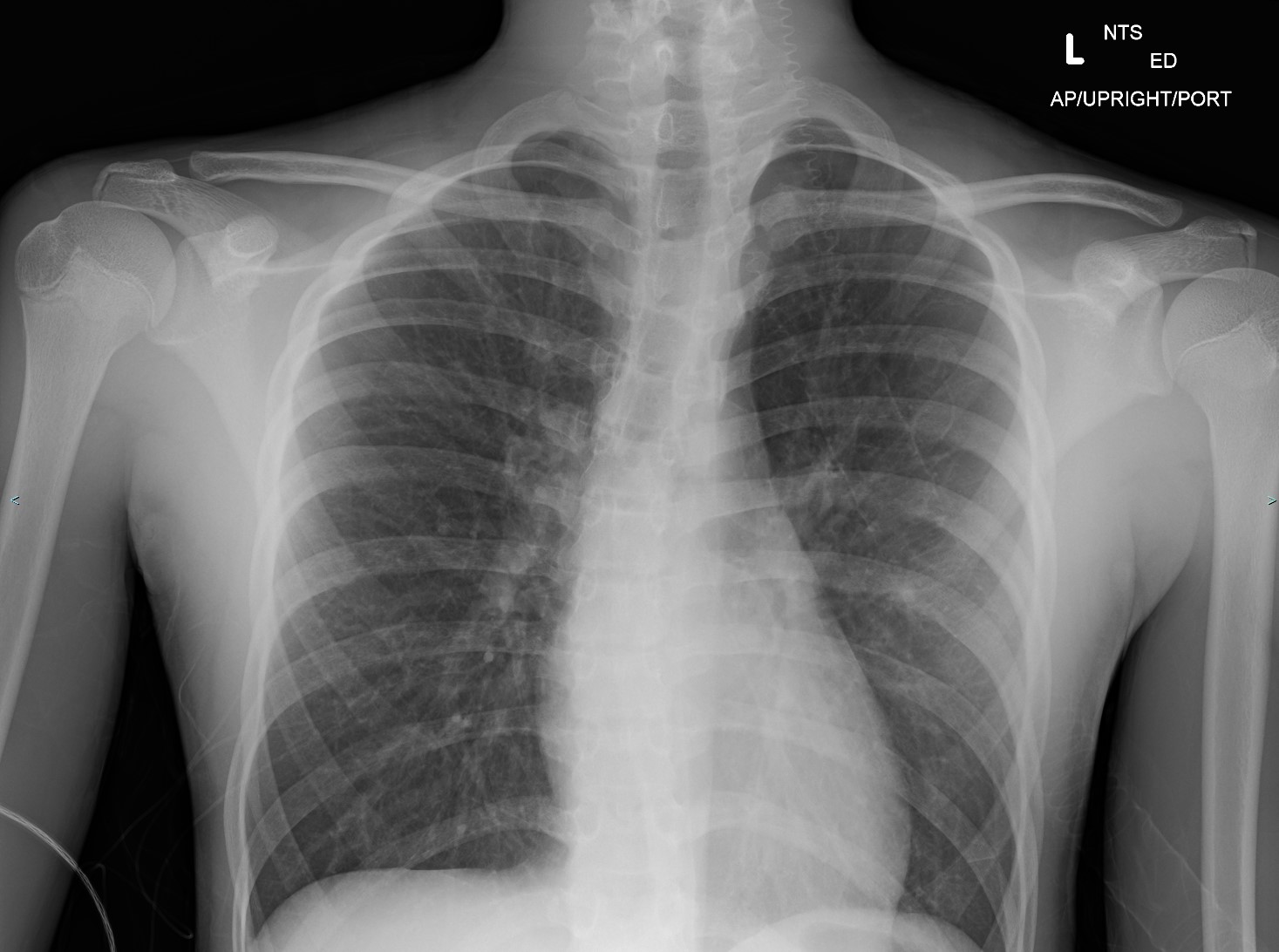
The chest radiograph can help identify the cause of poorly responsive asthma. Bronchiectasis, pneumonia, or pneumothorax are some pulmonary conditions that may cause asthma treatment refractoriness. Airway wall thickening and hyperinflation are usual findings in poorly controlled asthma (see Image. Asthma Radiography).
Point-of-care ultrasound can differentiate acute congestive heart failure, pleural effusions, pneumonia, and chronic obstructive pulmonary disease (COPD) from asthma exacerbation. This modality can also help assess hemodynamic status.[24]
ABG analysis can help evaluate oxygenation and ventilation, though the results should always be interpreted in conjunction with patient presentation. This test may also determine the presence of respiratory failure. Initial blood gas results may indicate respiratory alkalosis with hypoxemia (see Table. ABG Findings in Acute Asthma). Carbon dioxide partial pressure (pCO2) may falsely normalize as the condition progresses before respiratory acidosis and severe hypoxia develop. The normalization of pCO2 may indicate respiratory fatigue and thus should not be interpreted as clinical improvement. Patients in this state may require mechanical ventilatory support.[25] Mountain et al detected a 28% incidence of anion-gap metabolic acidosis caused by rising lactate in their study of 229 hospitalized patients with acute asthma.
Table. ABG Findings in Acute Asthma
| Clinical state |
pH status |
pCO2 |
pO2 |
| Early or mild asthma exacerbation |
Respiratory alkalosis |
Decreased |
Normal |
| Moderate exacerbation |
Respiratory alkalosis |
Moderate decrease |
Decreased |
| Impending respiratory failure (Status asthmaticus) |
Normal pH |
Normal |
Moderate decrease |
| Impending respiratory arrest |
Respiratory acidosis |
Increased |
Severely decreased |
Immunoglobulin E, skin tests, and radioallergosorbent tests can help identify the trigger in patients with suspected exposure to an unknown allergen. Atopy can complicate asthma management. The best way to control atopy is trigger avoidance.
Treatment / Management
Indications for Hospitalization and Intensive Care
Serial PEFR measurement is a practical and reliable predictor of severity and the need for hospitalization. Stein and Cole found that a significant PEFR improvement 2 hours after treatment predicted the need for hospitalization, even though initial PEFR on presentation did not. PEFR was noted to increase from 250 to 330 liters per minute in treated patients. Rodrigo and Rodrigo demonstrated a similar pattern in FEV1 with treatment response, although this parameter may not be the most practical to obtain at the bedside.[26][27][28]
Favorable initial treatment responses in status asthmaticus are visible symptom improvement sustained for at least 30 minutes after the last bronchodilator dose and a PEFR greater than 70% of the predicted value. On the other hand, less than 10% PEFR improvement or PEFR less than 40% of the predicted value is evidence of continuing clinical decline, warranting ICU admission. Intensive care should also be considered in the presence of increasing respiratory failure, mental status alteration, arrhythmia, cardiac or respiratory arrest, or complications like pneumothorax or pneumomediastinum. Patients with these conditions may also require aggressive resuscitation measures.
Patients who respond initially to treatment may benefit from a longer observation period in a clinical setting. Kelsen and colleagues found that patients treated for 2 hours or less in a facility had a 50% relapse rate. In contrast, only a 4% relapse rate was noted in individuals treated and observed for an additional 2 to 4 hours in a facility. Patients responding to asthma treatments must thus be observed further before deciding whether admission or discharge is the appropriate disposition afterward. The consensus for the observation duration varies between 4 to 6 hours.
The decision to hospitalize the patient may be based on both clinical and psychosocial grounds. For example, FEV1 or PEFR between 40% and 70% of the predicted value after initial treatment in the emergency room is considered an "inadequate response." Poorly responsive patients will need further stabilization. Hospitalization may also be considered in patients with poor psychosocial makeup or a hostile home environment with obvious exposure to triggers.
Pharmacological Interventions for Bronchoconstriction
Reducing bronchospasm and improving oxygenation and ventilation are the initial goals of asthma exacerbation treatment. Pharmacological agents are available for reducing lower airway obstruction.
β2-agonists
Short-acting inhaled β2-agonists are the first-line drugs for treating acute asthma exacerbation. Albuterol's greater β2 selectivity and longer action duration make it the preferred agent in this class over metaproterenol. Patient factors that can adversely affect β2-agonists' dose-response curves and action durations include preexisting bronchoconstriction, airway inflammation, mucus plugging, and poor patient effort. Larger and more frequent dosing may be necessary in the presence of these factors.
Initial treatment consists of 2.5 mg of albuterol (0.5 mL of a 0.5% solution in 2.5 mL normal saline) by nebulization every 20 minutes for 3 doses, followed by hourly treatments during the first several hours of therapy. Idris and colleagues demonstrated that even in patients with severe disease, 4 puffs of albuterol (0.36 mg) delivered with a metered-dose inhaler (MDI) and spacer were as effective as a 2.5 mg dose by nebulization. However, nebulization is still preferred in an emergency setting because it requires less supervision, coordination, and continued instructions.
An area that needs clarity is the appropriate mode of delivering inhaled medications in a ventilated patient. So far, consensus favors a higher dosage in intubated than nonintubated patients. However, debates exist about aspects like drug delivery mode (ie, by MDI or nebulization), ventilation mode, and connection site of the delivery device on the ventilator circuit.
The optimal delivery device has been a particularly polarizing point. Mcintyre and colleagues demonstrated that only 2.9% of a radioactive aerosol was deposited in the lungs when delivered by a small-volume nebulizer. Thus, the group advocated MDI use via an adapter attached to the ventilator circuit's inspiratory limb. However, a subsequent study by Manthous and colleagues refuted the McIntyre group's findings, demonstrating MDI's lesser effect on inspiratory flow-resistive pressure as opposed to nebulized albuterol.[29] Peak-to-plateau airway pressure gradient may be used as an indicator of bronchodilator effectiveness delivered in either mode. A 15% or greater decline in the gradient is considered a favorable response. Patients must be monitored for toxicity signs with repetitive doses.
Subcutaneous epinephrine and terbutaline have been used in the past but have fallen out of favor due to these drugs' toxic effects. Direct endotracheal epinephrine instillation also demonstrated a lack of efficacy in studies. However, intramuscular or intravenous epinephrine should be considered when aerosolized albuterol is ineffective or cannot be administered or if an allergen triggered the asthma attack.[30][31] Dosing is 0.3 to 0.5 mg intramuscular or 5 to 20 mcg every 2 to 5 minutes intravenously. Patients with hypotension or refractoriness to other treatments may be started on an epinephrine infusion at 0.1 to 0.5 mcg/kg/min.
Intravenous β2-agonists are not routinely recommended. However, these agents have been tried with some success in younger patients with status asthmaticus unresponsive to inhaled therapy.
Recent studies show a correlation between asthma mortality and inhaled β2-agonist use. Suissa and colleagues demonstrated that asthma mortality risk increases drastically when at least 1.4 canisters of inhaled β2-agonists are used monthly. The American Academy of Allergy and Immunology Executive Committee published a position statement on β2-agonist inhalational treatment in asthma.[32] The conclusions are as follows:
- Requiring more than 1 canister of β2-agonists a month should be considered a marker of severe asthma.
- Heavy or increased β2-agonist use warrants additional pharmacotherapeutic agents, such as corticosteroids.
- Long-acting β2-agonists can make asthma worse, but the available data do not allow for a definitive conclusion regarding this controversy.
- Patients currently using β2-agonists should slowly withdraw nonessential doses and use them only for rescue purposes.
Short-acting inhaled β2-agonists should not be withheld or underdosed during acute attacks regardless of the concerns about their long-term use. These agents remain the drugs of choice during acute asthma exacerbations.
Methylxanthines
Aminophylline and theophylline are bronchodilators with immunomodulatory effects, acting via phosphodiesterase inhibition and adenosine receptor antagonism. Despite these drugs' benefits, methylxanthines are associated with more adverse effects than β2-agonists. Methylxanthines are more likely to cause cardiac dysrhythmias than β2-agonists. Thus, these drugs are indicated mainly for individuals refractory to typical treatment. Patients should be monitored closely for signs of toxicity.[33]
Corticosteroids
Most available data support corticosteroids' distinct benefit in status asthmaticus in emergency settings.[34] Rowe et al's meta-analysis concluded that steroid use in the emergency department significantly reduced admission rates and relapses in the subsequent 7 to 10 days. The route of administration did not make a difference. Meanwhile, McFadden recommended a dose of 150 to 225 mg of prednisone a day or its equivalent to reach maximum therapeutic benefit based on available data.
Littenberg and Gluck also demonstrated a significant reduction in hospitalizations with a methylprednisolone dose of 125 mg intravenously in emergency room presentations. Available data support the administration of 60 to 125 mg methylprednisolone intravenously every 6 hours for the initial 24 hours of status asthmaticus treatment. Oral steroids are usually required for the next 10 to 14 days.
Physiologically, steroids reduce airway inflammation and mucus production, potentiate β2-agonist activity in smooth muscles, and reduce β2-agonist tachyphylaxis in patients with severe asthma.
Anticholinergics
Anticholinergics elicit variable responses in acute asthma exacerbation and have a somewhat underwhelming bronchodilatory role. However, these drugs may be useful in patients with β-blockade-induced bronchospasm or severe underlying obstructive disease with FEV1 less than 25% of the predicted value.
Bryant and Rogers demonstrated that nebulization with 0.25 mg of ipratropium bromide with 5 mg of albuterol administered resulted in greater FEV1 improvement than albuterol alone. The response time was also much faster than corticosteroids, with a detectable FEV1 change within 19 minutes.
Nebulized glycopyrrolate is also an alternative, although this agent is rarely prescribed in the United States. Available data and practice still recommend anticholinergics as second-line status asthmaticus agents when patients have an inadequate response to β2-agonist or steroids. The consensus choice is a 0.5-mg dose of ipratropium combined with albuterol administered by nebulization.
Magnesium sulfate
Magnesium inhibits calcium-mediated smooth muscle constriction, decreases acetylcholine release in the neuromuscular junction, and increases respiratory muscle force generation. Intravenous magnesium sulfate has been a useful adjunct in patients with β2-agonist-refractory acute status asthmaticus.[35] The benefit does not seem to isolate patients with low serum magnesium levels, although 50% of patients with acute asthma tend to present with hypomagnesemia.
Despite widespread use in emergency department settings, 2 large prospective studies failed to demonstrate any statistically significant lung function improvement when using this drug in severe asthma exacerbation. However, magnesium is relatively cheap and harmless. Women's increased responsiveness may be due to estrogen's ability to potentiate magnesium's bronchodilator effects. Hypotension and hyporeflexia are relatively infrequent at the commonly used dose of 2 g intravenously in 2 separate doses over 20 minutes.
Oxygenation and Ventilation
Oxygen supplementation and ventilatory support are routinely given to patients experiencing dyspnea. The different oxygenation and ventilatory approaches are explained below.
Heliox and oxygen
A true shunt in acute asthma has only 1.5% of the pulmonary blood flow. Therefore, oxygen may be given infrequently and at a low dose in status asthmaticus. Refractory hypoxemia in status asthmaticus should trigger a search for complications like pneumonia, atelectasis, or barotrauma.
Heliox is a 70:30 or 60:40 helium-oxygen mixture that decreases airway resistance and turbulence, work of breathing, and inspiratory muscle fatigue. This treatment has been demonstrated to reduce pulsus paradoxus and peak flow enhancement. However, the routine use of this modality is hindered by its prohibitive cost, infrequent indication, and need for recalibrating gas blenders and flow meters when used with mechanical ventilation.[36]
Noninvasive ventilation
Noninvasive ventilation (NIV) with continuous or bilevel-positive airway pressure may be used for ventilatory support in patients without significant encephalopathy and excessive secretions. These modalities also help avoid nosocomial infections and the need for anesthesia and sedation, which are endotracheal intubation-associated risks.
NIV is increasingly used in the first 24 hours at pressure support titrated to a respiratory rate below 25 per minute and tidal volume above 7 ml/kg body weight. However, NIV can produce suboptimal ventilation outside these values. Additionally, prolonged NIV increases the risk of complications like aspiration and facial pressure necrosis. Endotracheal intubation must be considered for patients requiring greater or prolonged ventilatory support.[37][38]
NIV's impact on asthma is not as well defined as in COPD. NIV has been shown to reduce the rate of invasive mechanical ventilation (IMV) and mortality associated with acute COPD exacerbation.[39] COPD and asthma's pathophysiological processes are similar. Therefore, NIV's benefit in status asthmaticus is likely similar.
Evidence suggests that NIV may reduce bronchodilator requirements and shorten ICU and hospital stays.[40][41] However, other studies show that NIV use does not change the IMV or mortality rates.[42][43] No high-power randomized controlled trials have investigated the use of NIV in asthma. However, NIV has been suggested to be a safe and well-tolerated intervention by other studies and should be considered early if a patient presents in extremis.[44]
Mechanical ventilation and sedation
The decision to intubate a patient with status asthmaticus is a clinical one and does not unequivocally require a blood gas assessment. Immediate indications for intubation include the following:
- Acute cardiopulmonary arrest
- Severe obtundation or coma
- Frank evidence of respiratory fatigue with gasping or inability to speak
- Persistent hypoxia despite multimodal treatment
Individuals who continue to deteriorate despite initial pharmacologic treatment require close bedside monitoring for possible intubation.[45] Clinical findings favoring intubation include the following:
- Increasing lethargy
- Increasing accessory muscle use
- Posture or speech changes
- Decreasing respiratory rate and depth
The choice of sedation agent is of paramount importance once a decision to intubate is made. The following are the options:
- Ketamine has sedative, analgesic, anesthetic, and bronchodilatory properties and has been increasingly recommended for emergency intubation in status asthmaticus along with succinylcholine. The usual dose is 1 to 2 mg/kg, given intravenously at a rate of 0.5 mg/kg/min to provide 10 to 15 minutes of general anesthesia without significant respiratory depression. Bolus dosing provides inconsistent systemic effects and is not recommended. The potential risks of this drug include the following:
- Hypertension and tachycardia due to sympathetic stimulation
- Seizure threshold lowering
- Increased laryngeal secretions
- Liver failure due to liver metabolism and accumulation
- Ketamine must be avoided in patients with uncontrolled hypertension, preeclampsia, raised intracranial pressure, epilepsy, and liver dysfunction.
- Propofol is an equally preferred initial anesthetic agent due to its rapid action onset, titratability, ability to cause deep sedation without paralytic drugs, and mild bronchodilatory effects. However, prolonged propofol administration elevates the risk of increased carbon dioxide retention by weakening the respiratory drive and reducing patient-ventilator synchrony. For ongoing sedation needs, lorazepam is preferred but used cautiously to minimize sedation to a level that maintains ventilator synchrony and allows response to stimulation.
- Paralytics are indicated when continuing ventilator asynchrony despite sedation and the risk of generating auto-positive end-expiratory pressure (auto-PEEP) or barotrauma. Atracurium is the agent of choice because of the lower risk of myopathy. However, this drug can cause bronchoconstriction due to histamine release. Vecuronium is an alternative with less bronchoconstriction risk.[46]
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) must be considered when hypoxia and acidosis persist despite mechanical ventilation.[47][48] ECMO is a highly specialized intervention requiring significant resources and support. ECMO is also associated with severe adverse effects, with hemorrhage being a primary concern. This modality may also cause limb ischemia. Mechanical problems may arise from the circuit.[49][50]
Antibiotics
Graham et al's randomized, double-blinded trial demonstrated that routine antibiotic use in status asthmaticus did not produce any difference in symptom score improvement, spirometry, or hospitalization length. However, antibiotics should be utilized if patients display clinical signs of infection. Respiratory culture specimens should be obtained early on if antibiotics are indicated.[51][52]
Differential Diagnosis
The differential diagnosis of status asthmaticus includes conditions presenting with severe respiratory distress that may or may not have wheezing on physical examination. Complications of asthma should also be considered.
- Pneumothorax may be differentiated by asymmetric breath sounds.
- Pneumomediastinum usually presents with a mediastinal crunch or crepitus around the neck or chest on examination.
- Tracheal obstruction or angioedema usually presents with inspiratory stridor; oral cavity or neck mass should be considered as a differential; prior history of tracheostomy or recurrent intubation may be due to tracheal stenosis.
- Foreign body inhalation, mucous plugging, or focal atelectasis conditions present with localized, not generalized, wheezing on auscultation.[53]
- Excessive dynamic airway collapse is associated with recurrent status asthmaticus and positive pressure ventilation use; occurs more frequently in adults
- Pneumonia: often presents with other adventitious sounds like rhonchi or lobar crackles.
- Patients with COPD often have chronic, worsening exertional dyspnea and a history of excessive cigarette smoking.
- Acute heart failure: may be differentiated by elevated cardiac enzymes or bedside echocardiography
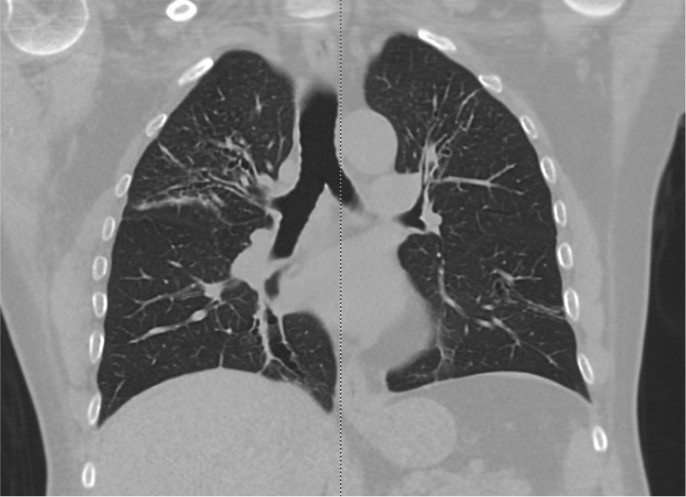
- Allergic bronchopulmonary aspergillosis may be distinguished by a positive Aspergillus skin prick test or IgE elevation and characteristic imaging findings (see Image. Allergic Bronchopulmonary Aspergillosis on Computed Tomography).
- Vocal cord dysfunction usually presents with inspiratory stridor and wheezing.
- Inhalational injury may precipitate asthma exacerbation; nasal and oral inflammation are evident.
- In neuromuscular diseases, such as myasthenia gravis, neurological examination usually reveals systemic neuromuscular involvement.
A thorough clinical evaluation and prudent use of diagnostic tests can help distinguish these conditions from status asthmaticus.
Prognosis
Status asthmaticus has a good prognosis if treated promptly, and comorbidities like congestive heart failure and COPD are absent. Meanwhile, Afessa et al reported a higher incidence of acidemia and carbon dioxide retention in nonsurvivors than survivors with acute status asthmaticus. The need for mechanical ventilation has also been reported as a poor prognostic factor.[54] A study by Adnet et al showed that neuromuscular blockade can increase the risk of post-intubation myopathy, ventilator-associated pneumonia, and duration of ICU stay in patients with status asthmaticus.
Complications
Acute hypotension beyond the initial sedation or post-intubation period needs immediate bedside intervention in patients with status asthmaticus.[55] Tension pneumothorax is the most time-sensitive pathology to be ruled out. The condition must be managed immediately by tube thoracostomy after diagnosis by bedside clinical examination, ultrasound, or chest x-ray.
Another frequent hypotension etiology in asthma patients on mechanical ventilation is dynamic hyperinflation, causing air trapping and auto-PEEP generation. Dynamic hyperinflation arises when the exhalation time is too short to permit complete emptying of the lungs. Consequently, the flow curve does not return to baseline. Delayed detection can result in increased pressure for a set tidal volume, increased risk of barotrauma, and increased intrathoracic pressure. Dynamic hyperinflation can decrease venous return and cause hypotension.
Patients manifesting with auto-PEEP should be transiently taken off the vent and their chest walls compressed to assist in emptying the lungs.[56] Exhalation time should be increased after resuming mechanical ventilation by reducing the tidal volume or respiratory rate. Some patients may need deeper sedation or paralysis. Ventilator-applied PEEP should be kept in moderation in patients with status asthmaticus due to the risk of barotrauma and hypotension.
Purposeful hypoventilation and permissive hypercapnia may be attempted in patients with status asthmaticus without raised intracranial pressure or severely depressed myocardial function. Moderate hypercapnia reduces dynamic hyperinflation and ventilation-perfusion heterogeneity. The goal is to maintain a pH greater than 7.5, which is generally well tolerated, rather than a target pCO2.
High peak pressure with stable plateau ventilator pressure should also prompt efforts to clear the airway and endotracheal tube of secretions. Patients with status asthmaticus tend to develop thick and tenacious secretions. A larger lumen endotracheal tube (French 7.5 or 8) is preferred due to the high airway resistance and need for airway clearance.
Other commonly reported complications are electrolyte abnormalities, hypotension, and dysrhythmias.[57] Severe hypotension and respiratory acidosis in refractory cases have resulted in myocardial infarction, cardiac arrest, hypoxic and anoxic encephalopathy, toxicity from medications, and death.
Consultations
Patients with status asthmaticus will require intensive care management. Specialists often involved in the care of these individuals include intensivists and pulmonologists. Social services should also be consulted to assist with social determinants, such as the ability to afford and access medication.
Deterrence and Patient Education
Exacerbation prevention is the best approach to asthma management. Trigger avoidance and treatment compliance are the best measures to prevent status asthmaticus. Environmental management is essential in patients with environmental allergies. Individuals with a greater exacerbation risk, such as people in extremes of age or with poor access to medications, should be identified. Inpatient education by trained laypeople has been found to improve inhaler management compliance and post-discharge care.[58]
Pearls and Other Issues
The key points to remember when evaluating and managing status asthmaticus are listed below.
- Exacerbation prevention is the best approach to asthma management. Patient education should focus on trigger avoidance and treatment compliance—the best preventive strategies against acute asthma exacerbation and complications like status asthmaticus. Patient factors predisposing to asthma exacerbation must be investigated.
- Recognizing early signs and symptoms of an asthma attack is crucial for prompt intervention and better outcomes. Acute dyspnea, coughing, and chest pain in a patient previously diagnosed with asthma could be signs of exacerbation. Refractoriness to the usual treatments may herald status asthmaticus and must be investigated in a clinical facility.
- Inhaled β2-agonists are the first-line agents for asthma exacerbation. Oxygen supplementation is also critical to management. Other pharmacotherapeutic drugs for asthma exacerbation include corticosteroids, methylxanthines, anticholinergics, and magnesium sulfate.
- The acute episode's trigger must be investigated and removed. Infections, allergies, and inhalational injuries are some possible causes. Antimicrobials must be given if infection is the likely cause, but these medications must not be routinely administered without infection symptoms.
- Patients with status asthmaticus often need ventilatory support. Noninvasive modalities must be considered first for patients without significant encephalopathy or secretions. However, a low threshold for endotracheal intubation should be maintained.
- Ketamine, propofol, and paralytics must be considered for sedation during intubation.
- ECMO may be considered in individuals who do not improve with mechanical ventilation and if all other potential causes of respiratory failure have been addressed, such as infection.
- Management decisions should be individualized for every patient.
Enhancing Healthcare Team Outcomes
Status asthmaticus management requires an interprofessional approach. Emergency medical services or emergency medicine physicians may provide initial evaluation and treatment, depending on the location where the patient is first encountered. Nurses on staff can help stabilize the patient, administer medications, and coordinate care. Radiologists can interpret the imaging findings and help guide management.
ICU admission and mechanical ventilation will require the services of intensivists, pulmonologists, and respiratory therapists. Other specialists, such as infectious disease specialists, cardiologists, and allergologists, may be involved if concomitant conditions need to be managed. Occupational therapists can help patients regain functional independence when nearing hospital discharge. Social workers can help patients and their families navigate the healthcare system for resources and help devise recurrence prevention strategies. Pharmacists can help with medication management.[59][60]
The primary care physician or pediatrician can monitor the patient on follow-up and educate families about managing the illness at home. Patients should be well-versed in detecting early warning signs of an attack and always be prepared for self-treatment based on their asthma action plan. A 20% drop in PEFR below the predicted value or personal best is an objective indicator of exacerbation. Patients with a history of anaphylaxis or sudden asphyxic asthma presentation should also be equipped with Epipen for immediate subcutaneous use if needed.
Health systems across the United States are endorsing outpatient pharmacies' role in monitoring patient compliance and detecting an increase in disease severity based on prescription refills. Bluetooth-enabled monitors on inhaler devices may help healthcare providers remotely monitor rescue inhaler needs and the appropriateness of inhaler-use techniques. An example of a similar sensor-enabled smart inhaler is the San Francisco-based propeller health device.[61]
Overall, the outcomes are good when a patient with an acute asthma exacerbation is brought to the emergency room and quickly managed according to a streamlined protocol.[62] However, the outcomes in patients requiring mechanical ventilation vary from moderate to severe.[63] Mortality is not uncommon in these patients. Nosocomial pneumonia while on mechanical ventilation is the chief reason for the high morbidity and mortality. Thus, educating the family regarding exacerbation prevention measures and immediate medical consultation for asthma exacerbation are crucial to managing patients with asthma.