Continuing Education Activity
Spinal cord ischemia and infarction occur less frequently than cerebral infarction but result in significant mortality, disability, and reduced quality of life in survivors. Modern imaging techniques and refined diagnostic criteria have increased awareness of spinal cord ischemia and enhanced the current knowledge of its pathophysiology. With prompt clinical recognition and judicious use of diagnostic studies and comprehensive clinical follow-up, occupational therapy, and rehabilitation, many patients can experience improved functional outcomes. This activity reviews the pathogenesis, diagnosis, evaluation, and treatment of spinal cord ischemia and emphasizes the role of the interprofessional team in improving care for patients with this condition.
Objectives:
- Summarize recent evidence-based diagnostic criteria for acute spinal cord ischemia.
- Review current theories regarding the mechanism of spinal cord ischemia.
- Outline the best imaging tools to evaluate for spinal cord ischemia.
- Describe the role of interprofessional teams in collaborating to prognosticate recovery from spinal cord ischemia.
Introduction
Spinal cord ischemia and infarction occur less frequently than cerebral infarction but result in significant mortality, disability, and reduced quality of life in survivors. Modern imaging techniques and refined diagnostic criteria have increased awareness of spinal cord ischemia and enhanced our understanding of its pathophysiology. With prompt clinical recognition and judicious use of diagnostic tests including imaging studies, many patients can experience good functional outcomes with comprehensive clinical follow-up, occupational therapy, and rehabilitation.
Etiology
Acute spinal cord ischemia (ASCI) can occur in a range of clinical settings, but a significant portion of documented cases occur in the setting of acute aortic pathology or aortic surgery. It is thus useful to classify etiologies as spontaneous or periprocedural.[1] Nonetheless, despite a thorough investigation, a causative source cannot be identified in a significant share of patients.[2][3][4]
Spontaneous Causes of Spinal Cord Ischemia
As with cerebral ischemia/infarction, the TOAST classification system provides a useful schema for developing a differential diagnosis in spinal cord ischemia.[5] Strokes may be attributable to large vessel atherosclerosis, cardioembolism, small-vessel occlusion (lacunar infarction), other/unknown etiologies. Aortic pathology is the most common source of spinal cord ischemia. Approximately 1% of patients presenting with acute Type A aortic dissection will have spinal cord stroke.[6] A case of thoracoabdominal aortic aneurysm, with or without associated dissection, is also associated with spinal cord ischemia.[7] In one larger study of ASCI, 33% of cases were attributed to atherosclerotic disease, 16% to aortic pathology, and 16% to degenerative spine disease.[8] Although less common, embolic stroke can occur from aortic atheroma, myxoma, or infectious valvular vegetation. A special case of embolic spinal cord infarction occurs to mobilized disc material in so-called “fibrocartilaginous emboli” (FCE), which may occur with acute disc herniation. Large osteophytes and spinal stenosis have also been associated with acute cord ischemia when paired with abrupt motion.[9]
Cervical vessel injury which results in interrupted flow to the upper anterior spinal artery can arise in the setting of trauma, abrupt head-turning, cocaine abuse, and chiropractic manipulation.[1][10] A panoply of less common causes of spontaneous cord ischemia has been described including anterior spinal artery (ASA) embolism, arteriovenous malformation, sickle cell anemia, lupus erythematosus, epidural hematoma, and decompression sickness.[3][9]
Iatrogenic Causes of Spinal Cord Ischemia
A significant number of cases of spinal cord ischemia occur in the periprocedural setting. Up to 45% of all reported cord infarctions in one case series were attributed to an iatrogenic cause.[2]
Aortic surgery has long had recognition as a risk factor for spinal cord stroke. Spinal cord ischemia can occur during both cross-clamping and de-clamping, the latter due to abrupt reperfusion of the viscera and resultant hyperperfusion of radicular arteries.[10] The risk of spinal cord ischemia resulting in permanent deficit following aortic grafting is substantial, reported between 0.3 and 6.5%.[11] The duration of cross-clamp time, pre-existing vascular risk factors, and length of the repaired aortic segment contribute to risk.
Cord infarction/ischemia following percutaneous procedures is a rare but devastating complication reported following celiac ganglion block, epidural steroid injection, intra-aortic balloon pump, and lumbar epidural catheter placement.[10][12][9] Unintentional embolization during spinal angiographic embolization procedures can also occur. Orthopedic lumbar surgery can cause infarction as a result of the microembolization of the disc material.[3][7]
Epidemiology
Spinal cord ischemia and infarction are uncommon relative to cerebrovascular stroke, constituting an estimated 0.3 to 1% of all strokes.[13] Acute spinal cord ischemia syndrome (ASCIS) is also rare among myelopathies, accounting for an estimated 5 to 8%.[8] Both sexes appear to be affected equally.[3][9] In many case series, affected patients had multiple traditional cardiovascular risk factors including hypertension, diabetes, and evidence of large-vessel atherosclerosis.[2][4][9][14]
Pathophysiology
Vascular Anatomy of the Spinal Cord
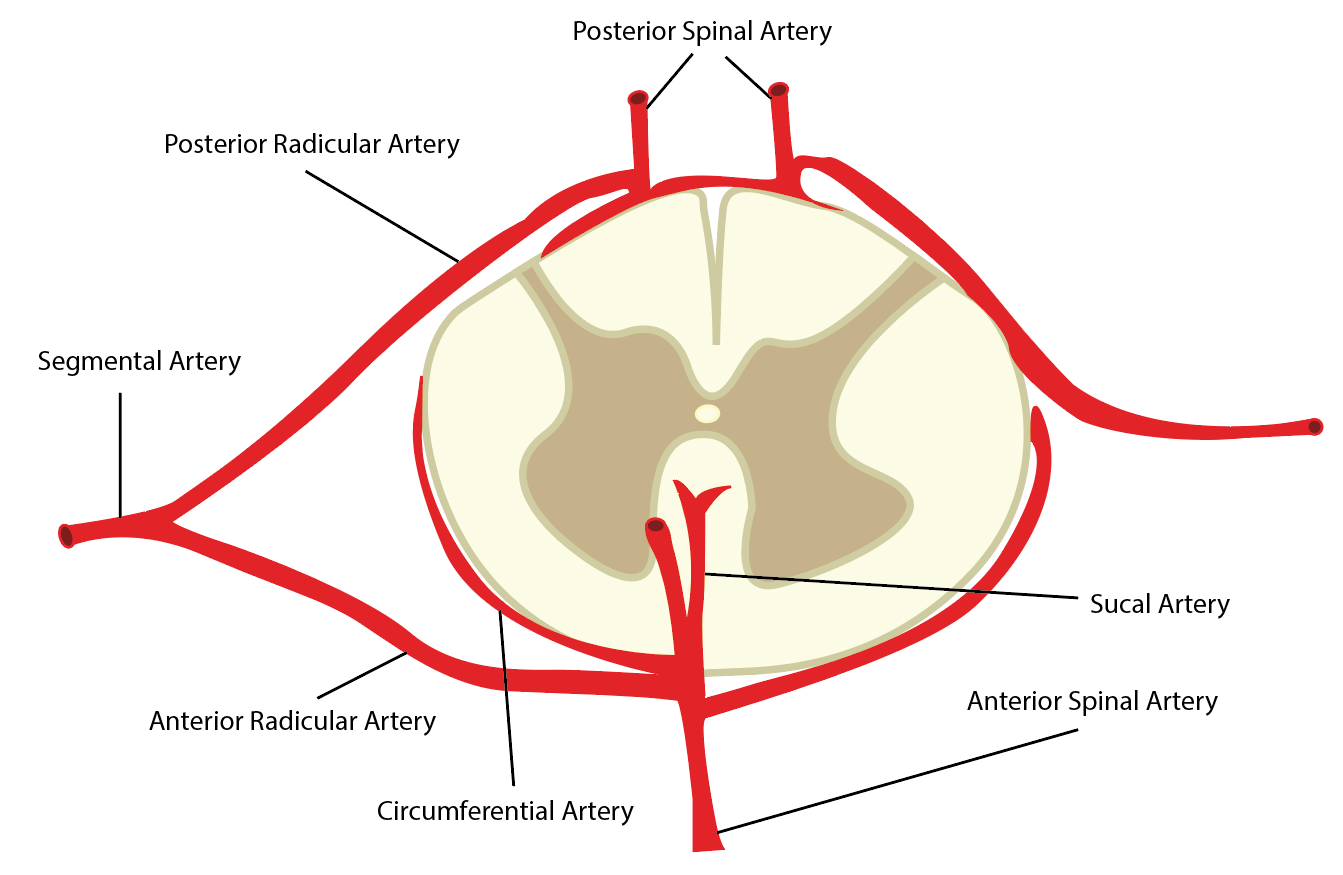
Vascular irrigation of the spinal cord is complex and shows significant inter-individual variation, particularly at the thoracolumbar levels.[15] The anterior spinal artery (ASA) is the dominant supplier of the cord. Originating at the level of the medulla at the vertebrobasilar junction, the ASA receives auxiliary supply from an inconsistent array of radiculomedullary arteries which in turn arise directly from the descending aorta, typically on the left. After bifurcating into anterior and posterior rami, the posterior rami subdivide further into spinal arteries followed by anterior and posterior radicular branches. Typically, some ten segmental arteries contribute to ASA although the number is quite variable.[15] The paired posterior spinal arteries (PSA) form a heterogeneous meshwork of anastomoses (spinal arterial plexus) with the ASA and receive supply from posterior radicular branches. ASA and PSA branches converge to form an arterial plexus, the vasa coronae, from which radially oriented perforator branches arise to supply the central cord. Blood flow in the ASA is uniformly caudal whereas the paired PSA may show bidirectional flow.
The major radicular artery of Adamkiewicz (AA) is the principal vascular source of the lower segments of the ASA. The artery of Adamkiewicz most frequently arises on the left between T7 and T12 levels and supplies roughly one-fourth of the entire cord in most patients.[9][15]
Some have divided the cord supply into four semi-distinct territories; the first extends from C1 to T3 and derives flow predominately from the vertebral arteries. The second region from T3 to T7 is often supplied by a left-sided intercostal artery. The third region receives supply from the artery of Adamkiewicz (T8 to T12, often extending to the conus). A 4th region including the conus sometimes derives flow from an internal iliac branch (Desproges-Gotteron artery).[3] The deep perforator vessels comprising the end arteries of the radiculomedullary feeders are termed “sulcocommisural” branches. Due to high perfusion overlap in the cervical and lumbar levels of these two systems, focal infarction due to isolated radiculomedullary vessel occlusion is unlikely.[15]
A potential watershed zone of less robust anastomoses exists between T4 and T8, rendering these levels susceptible to transient perfusion abnormalities. However, aggregated case series show the distribution of spinal cord strokes does not corroborate this hypothesis, and some have suggested that relative hypovascularity of the mid-thoracic cord reflects metabolic parsimony due to relatively decreased oxygen demand at these levels relative to other cord regions.[15]
Mechanisms of Spinal Cord Ischemia
Ischemia of the spinal cord may arise from local vascular injury or global hypoperfusion. In the case of acute aortic dissection, injury to the cord occurs due to decreased flow related to increased aortic impedance from the flap itself or via an extension of the flap resulting in occlusion of a critical intercostal feeder.[6] A larger deficit may occur if the dissection involves the artery of Adamkiewicz, which supplies the levels of T10 to L1 in most patients.[13]
As with cerebral blood flow, spinal cord vasoregulation is dependent on pH and pCO2. Ischemia triggers an inflammatory response and NMDA-mediated neuronal excitotoxicity. Most cell death occurs at 48 hours following onset, predominately via controlled apoptosis.[15]
Relative cord blood flow is proportional to gray matter density and varies throughout the cord levels. Analogous to the Monro-Kellie hypothesis which describes the zero-sum volume relationship of neural tissue, blood, and CSF in the intracranial space, spinal cord perfusion is affected by relative CSF pressure. This observation has led to the clinical practice of prophylactic CSF drainage during aortic surgery to preserve cord perfusion.[11][16]
Spinal venous ischemia/infarct is uncommon and often indolent. Venous hypertension and chronic ischemia classically correlate with spinal arteriovenous malformations which result in distension of the perimedullary venous plexus.[17] The resultant subacute myelopathy may go unrecognized or mistaken for spondyloarthropathy. This pattern of injury may also be present in decompression sickness related to scuba diving in which nitrogen bubbles can obliterate venules.[12][10] Other mass-occupying lesions in the spinal canal can including epidural hematoma and abscess mimic venous ischemia clinically.[8][10]
History and Physical
Careful documentation of patient history including the timeline of symptoms, plausibility of alternative diagnoses should be obtained and serial neurologic exams to document onset, nadir, and recovery of neurologic function are essential.
While clinical presentation varies by the affected vessel and topography of cord ischemia, the classic acute presentation is painless paraplegia and urinary retention. A rapid decline of function (within 12 hours of onset) with a severe neurologic deficit is characteristic; gradual onset of symptoms is highly suggestive of alternative etiology.[1] However, up to 17% of patients have biphasic syndrome with transient symptoms and later deterioration.[18] Some patients may experience paresthesia or dysesthesia below the level of injury (~29%).[9] A small portion of patients may present after an inciting maneuver such as Valsalva or abrupt change in position.[3]
The most common neuroanatomic manifestation of ASCI is ASA syndrome which is characterized by injury of the ventral two-thirds of the cord with sparing of the PSA-supplied dorsal columns. Full ASA syndrome comprises flaccid paraplegia, loss of spinothalamic pain and temperature sensation, and autonomic dysfunction of bowel and bladder. Injury of the anterior horn neurons, when present, results in lower motor signs such as absent hyperreflexia and abnormal electromyography (EMG).
Focal ASA ischemia restricted to smaller distributions can present with characteristic loss-of-function germane to the affected level. Injury involving the lower cervical levels (C3 to C5) may result in diaphragmatic paralysis. Cervical ASA syndrome may also present with quadriparesis and may correlate with loss of dorsal column function due to medial lemniscus involvement in which case, light touch, and proprioception are also affected in contrast to lower forms of ASA syndrome.[14] Mid-thoracic (T4 to T9) lesions involving the splanchnic autoregulatory system can produce orthostatic hypotension or spinal dysautonomia. Focal injury of the sacral cord and lumbar nerve roots presents with isolated areflexia of the urinary bladder, bowel, and lower limbs. When ischemia is present at multiple levels, as with hypoperfusion, vascular myelopathy may be challenging to differentiate from other etiologies of myelopathy.[12] Early on, flaccid paralysis is the dominant clinical finding due to spinal “shock.” However, over time, hyperreflexia, spasticity, and extensor plantar reflexes prevail.
Brown-Sequard syndrome (BSS) is the eponymous description of spinal cord hemisection, most frequently associated with trauma. BSS may occasionally be seen in ischemia/infarction with an injury to the sulcocommisural perforator which can result in ipsilateral hemiparesis and contralateral loss of pain and proprioception.[19][9]
A subset of patients present with stuttering, repetitive symptomatic episodes, termed spinal cord transient ischemic attacks (STIA).[12] An STIA occurring with a postural change in the setting of advanced spondyloarthropathy or discogenic disease may be due to neuro foraminal compression of radicular feeders. Clinicians should consider FCE in patients who present with acute back pain and delayed paraplegia with imaging showing co-existing discogenic disease when atherosclerotic sources are absent.[20] Spinal claudication is the term used to describe exertional myelopathy syndrome which may be related to coexisting spinal stenosis and vascular compression.[21] Patients may describe lower extremity heaviness with or without pain and sphincter dysfunction and relief of symptoms with rest. Cervical claudication has also been described in patients engaging in upward reaching or highly repetitive activities, plausibly due to mechanical tension on the cervical cord supply.[3]
A rare but highly specific syndrome known as “surfer’s myelopathy” has been described in novice surfers presumably due to prolonged prone positioning which invokes a tension/avulsion mechanism of cord devascularization.[22]
Evaluation
Magnetic Resonance Imaging (MRI)
MRI is the imaging modality of choice for suspected spinal cord ischemia although it is worth noting that MRI may appear entirely normal in up to 24% of acutely symptomatic patients.[1] MRI imaging protocols should include both sagittal and axial T1 and T2-weighted sequences, post-contrast imaging, and diffusion-weighted imaging (DWI).[17]
DWI MRI is exquisitely sensitive for detecting cord ischemia and shows a characteristic high signal, indicating cytotoxic edema and reduced free water diffusivity.[10] Swelling of the affected segment typically occurs within 8 hours of injury however DWI-negative cord infarction can be seen in the hyperacute setting (less than 24 hours) and should not exclude the diagnosis when other evidence is supportive.[1] DWI of the cord is technically challenging and has only recently become widespread. Thus many older clinic-radiographic correlations did not incorporate DWI imaging.
Gadolinium enhancement can also distinguish acute cord ischemia, in which the blood-brain barrier is intact (absent enhancement) from non-vascular etiologies which may enhance (intramedullary tumor etc.). The normal evolution of cord infarction results in an enhancement in the subacute phase, approximately 3 to 4 days after the initial insult.[10]
A variety of characteristic MRI “signs” have been described which can support the diagnosis of spinal cord ischemia in the appropriate clinical setting. T2-weighted MRI may show a well-circumscribed high signal lesion at the site of ischemia. In the sagittal plane, this appears as a “pencil-like” zone of signal abnormality.[10] Sagittal T1-weighted imaging may show segmental cord swelling or focally elevated signal thought to represent hemorrhagic transformation.[10][17] The “owl’s eye” sign describes the appearance of symmetrical T2 signal abnormality of the anterior horn neurons which are metabolically susceptible to global ischemia.[3] While this finding strongly correlates with ASA syndrome particularly at the cervical level, it can be present in a number of mimic pathologies including viral encephalomyelitis, poliomyelitis, and paraneoplastic myelopathy.[10] The “positive anterior cauda” sign has also received support as a feature of cord ischemia. This appearance shows as asymmetrical enhancement of the anterior cauda equina nerve roots occurring in thoracolumbar ischemia. This phenomenon may be due to differential perfusion of the anterior and posterior nerve roots.[10] Although uncommon, MRI evidence of acute infarction of the adjacent vertebral body and disc is considered highly specific for ischemic/vascular etiology of a focal spinal cord lesion.[23]
Other Imaging Modalities
The role of computed tomogram (CT) is limited in evaluating suspected spinal cord ischemia due to low sensitivity though it may serve to rapidly assess for treatable causes such as epidural hematoma or displaced fracture compromising vascular flow.[17] Similarly, CT myelography, which was historically used to evaluate the contour of the thecal sac and nerve roots, has largely been replaced by the widespread use of MRI.
Digital subtraction angiography (DSA) is rarely indicated in ASCI. When clinical features of venous/congestive myelopathy are present, or characteristic MRI findings (dilated vascular flow voids) are suggestive, DSA may be indicated to confirm suspected spinal arteriovenous malformation, fistula, or aneurysm.[12]
Lumbar Puncture
CSF fluid analysis is generally not helpful in the initial workup of spinal cord ischemia. CSF may variably show elevated protein concentration which is nonspecific.[3][1] However, CSF analysis may help diagnose mimic pathologies such as viral myelitis and neuromyelitis optica when there is a high index of suspicion.[10]
Emerging Techniques
A range of modern MRI techniques has emerged with potential applications in the evaluation of spinal cord injury and ischemia. Diffusion tensor imaging (DTI), magnetization transfer imaging (MT), the myelin-water fraction (MWF), MR spectroscopy (MRS), and functional MRI (fMRI) have been studied in spinal cord imaging and have potential applications in ischemia. DTI, which measures directional diffusion of water molecules, can approximate axonal integrity and, according to a recent systematic review, shows the greatest promise in imaging cord microstructure integrity.[24] While these techniques are promising, none yet play a routine role in the current diagnosis and management of spinal cord ischemia.[17]
Treatment / Management
In contrast to cerebral ischemic infarction, in which guidelines for management and pre- and post-hospital care are well-established, no consensus guidelines exist for the management of acute spinal cord ischemia.[25] Given the rarity and heterogeneity of cord ischemia, treatment decisions should be based on individual patient circumstances, aiming to minimize short-term complications and long-term morbidity.
Surgical Considerations
Specific etiologies of spinal cord ischemia may be amenable to surgical intervention. Open decompression of epidural abscess or hematoma in the acute setting may prevent secondary vascular injury and long-term neurologic deficit.[26] Given the prevalence of cord ischemia following aortic surgery, much work has been done to investigate perioperative management and optimization of patients undergoing endovascular and open aortic surgery. Lumbar CSF drainage is now a widely accepted preventative measure that is believed to augment spinal cord perfusion pressure during fluctuations related to anesthesia and aortic manipulation.[27] CSF drainage is generally well-tolerated although it carries some risk including subdural hematoma.[16]. Selective CSF and epidural hypothermia protocols, typically used in conjunction with CSF drainage, may also prevent postoperative ischemia.[28] Intraoperative monitoring of motor and somatosensory evoked potentials has also proven useful in reducing ischemic events.[29] The importance of preserved cord perfusion has gained support from studies of intra-operative blood pressures in which patients who maintained mean arterial pressures (MAP) above 55mm Hg had associations with few neurologic deficits.[7][30]
Medical Management and Considerations
Unfortunately, there are scarce data on the medical management of spinal cord ischemia. Reports exist of treatment with thrombolysis, but the generalizability of this therapy and its associated risks are not certain.[31] Patients with acute spinal cord injury may benefit from early administration of corticosteroids in terms of functional recovery although whether this benefit extends to patients with vascular cord ischemia remains uncertain.[32] Anticoagulation and antiplatelet agents have been used in cases with suspected atherosclerotic etiology although prospective trials are lacking.[1]
Differential Diagnosis
The differential diagnosis includes clinical syndromes and imaging findings that overlap or share similar features to true spinal cord ischemia. Differentials include a variety of myelopathies which can be categorized as compressive, infectious, inflammatory, or nutritional (as in the case of posterior column disease related to B12 deficiency). Compressive etiologies are easily diagnosed with routine imaging and may include extramedullary tumor, hematoma, abscess, or degenerative bony remodeling.[9] Infectious etiologies require characteristic history and supportive laboratory studies and may include HTLV1, HIV, Varicella, and progressive multifocal leukoencephalopathy (PML). Inflammatory/autoimmune conditions include transverse myelitis and multiple sclerosis.[12] The longitudinal cord lesions seen in neuromyelitis optica can also mimic cord ischemia.[17]
Diagnostic criteria have recently proposed by Zalewski et al. based on experience with a cohort of 133 ASCI patients compared to patients with non-ischemic myelopathy.[1] The proposed SCI diagnostic criteria incorporate history/presentation, MRI findings, CSF analysis, and the probability of alternative diagnoses. The resultant schema classifies patients as having definite, probable, or possible SCI which may further subdivide as spontaneous or periprocedural. In a validation cohort of 280 patients with non-ischemic diagnoses, only 3.2% met criteria for possible SCI, and none classified as definite or probable suggesting that the presented criteria are highly specific.
Staging
The American Spinal Injury Association (ASIA) scoring system is used commonly used to grade the severity of deficit following spinal cord ischemia.[33][2] This system summarizes the motor and sensory deficits by the level of injury using a six-point scale (0 = complete paralysis, 5 = active movement against full resistance) and provides a multidimensional evaluation of functional outcome in terms of self-care, sphincter control, mobility, locomotion, communication, and social cognition. Dependence on caregiver assistance is also incorporated. The resulting 5 level score (A-E) provides a standardized method of assessing spinal cord injury. ASIA scores A and B are considered a severe impairment, C is moderate, D is mild, and E is no impairment/normal function.
Prognosis
Many patients experience significant motor recovery following cord ischemia/infarction although the prognosis is challenging to predict. In one series, approximately 42% of patients showed some or marked improvement whereas 36% showed little or no improvement. The series also observed that patients with sparing of knee extension and hip abduction might have a better prognosis.[9] In another case series, 68% of patients had a maximum deficit at one hour following injury, and the nadir of function was highly predictive of long-term outcomes.[2] Nedeltchev et al. found that, at a mean follow-up of 4.5 years, 41% of patients were able to walk independently and another 31% with ambulatory support devices. Despite this relatively high rate of post-infarct ambulation, the reported long-term mortality in this cohort was 9%.[8]
Patients with ASA syndrome have the worst prognosis relative to other patterns, presumably due to the more considerable extent of cord infarction. Reports indicate that older age at presentation and severity of deficit at nadir are predictive of poor outcomes.[4]
A recent study with long-term follow-up (average over 7 years) compared functional outcomes of patients with cerebral and spinal cord infarction and found that spinal patients had higher rates of employment and lower mortality but higher rates of chronic pain and lowered functional independence (modified Rankin scale).[18] These authors have argued that long-term follow-up unmasks better outcomes, for example, noting that that two-thirds of their patients were able to walk at maximum follow-up whereas only 50% could walk at 1 week, which suggests that older studies, which included shorter follow-up, may overestimate functional impairment.
Complications
The chief sources of disability and morbidity following cord infarct are lower extremity paresis and urinary retention.[13] These can significantly reduce the quality of life and life expectancy in the long run due to recurring complications such as urinary tract infections and decubitus ulcers. Among surviving patients, an estimated 42% will require long-term wheelchair use and 54% require catheterization to manage urinary retention.[2]
Deterrence and Patient Education
Patients are encouraged to seek guidance and support from advocacy groups for patients and family members with spinal cord injury. The Christopher and Diana Reeve Foundation provides resources for both patients and caregivers of people with spinal cord paralysis including information on insurance, disability, and rehabilitation services. Online community support is also available. Additional resources include the Paralyzed Veterans of America (PVA.org), which promotes research, awareness, community support, and adaptive sports competitions. The United Spinal Association (unitedspinal.org) also serves as a large advocacy organization online and is replete with resources for patients and those hoping to support spinal injury patients with donations, peer support, and political advocacy for disability rights.
Enhancing Healthcare Team Outcomes
Care for patients with spinal cord ischemia and infarction requires comprehensive, interprofessional healthcare provider engagement. Neurology and Emergency Medicine providers must recognize the often atypical symptoms of cord ischemia and initiate appropriate triage and workup. Neuroradiologists must optimize spinal cord MRI imaging protocols and ensure symptomatic patients receive an expedited radiologic evaluation. When indicated, neurosurgical treatment of reversible causes of cord ischemia should occur promptly following diagnosis. Given the significant overlap of aortic pathology and spinal cord ischemia, many cases of spinal cord ischemia may first present to vascular surgeons or cardiologists; thus interprofessional communication and effective consultation services are essential.
Physiatrists and non-physicians including specialty-trained rehabilitation nurses also play a critical role in the recovery phase following spinal cord infarction. Patients should be counseled on prognosis and appropriately referred for occupational and physical rehabilitation services. Licensed social workers are needed to assist patients during the road to recovery by helping navigate the post-hospitalization rehabilitation process including insurance claim processing and obtaining support devices. The cumulative efforts of the entire care team can optimize the patient’s chance for functional recovery.