Continuing Education Activity
Congenital melanocytic nevus (CMN) is a benign skin lesion typically present at birth, characterized by nests of melanocytes that may extend into the dermis and, in some cases, deeper structures such as muscle or the central nervous system. CMN is categorized by size: small (<1.5 cm), medium (1.5–20 cm), and large or giant (>20 cm projected adult diameter). Larger lesions, particularly giant congenital melanocytic nevi, carry a higher risk of malignant transformation into melanoma, although this risk remains variable. These lesions may present with a wide range of clinical features and can be associated with extracutaneous conditions, particularly in cases of larger or giant nevi.
This course provides healthcare professionals with a comprehensive understanding of the epidemiology, genetics, clinical presentation, and management strategies for CMN. Participants learn to identify risk factors, recognize extracutaneous associations, and determine appropriate treatment plans, including surgical and nonsurgical options. Emphasis is placed on interprofessional collaboration, involving dermatologists, surgeons, oncologists, and genetic counselors to develop individualized care plans that optimize patient outcomes, minimize complications, and address the psychosocial impacts of these lesions.
Objectives:
Identify the clinical features of congenital melanocytic nevi, including size classifications and associated risk factors for malignancy.
Differentiate between small, medium, and large/giant congenital melanocytic nevi and their respective implications for surveillance and management.
Screen for neurocutaneous melanosis in patients with large or giant congenital melanocytic nevi using appropriate imaging techniques.
Collaborate with the interprofessional team to educate, treat, and monitor patients with congenital melanocytic nevi to improve patient outcomes.
Introduction
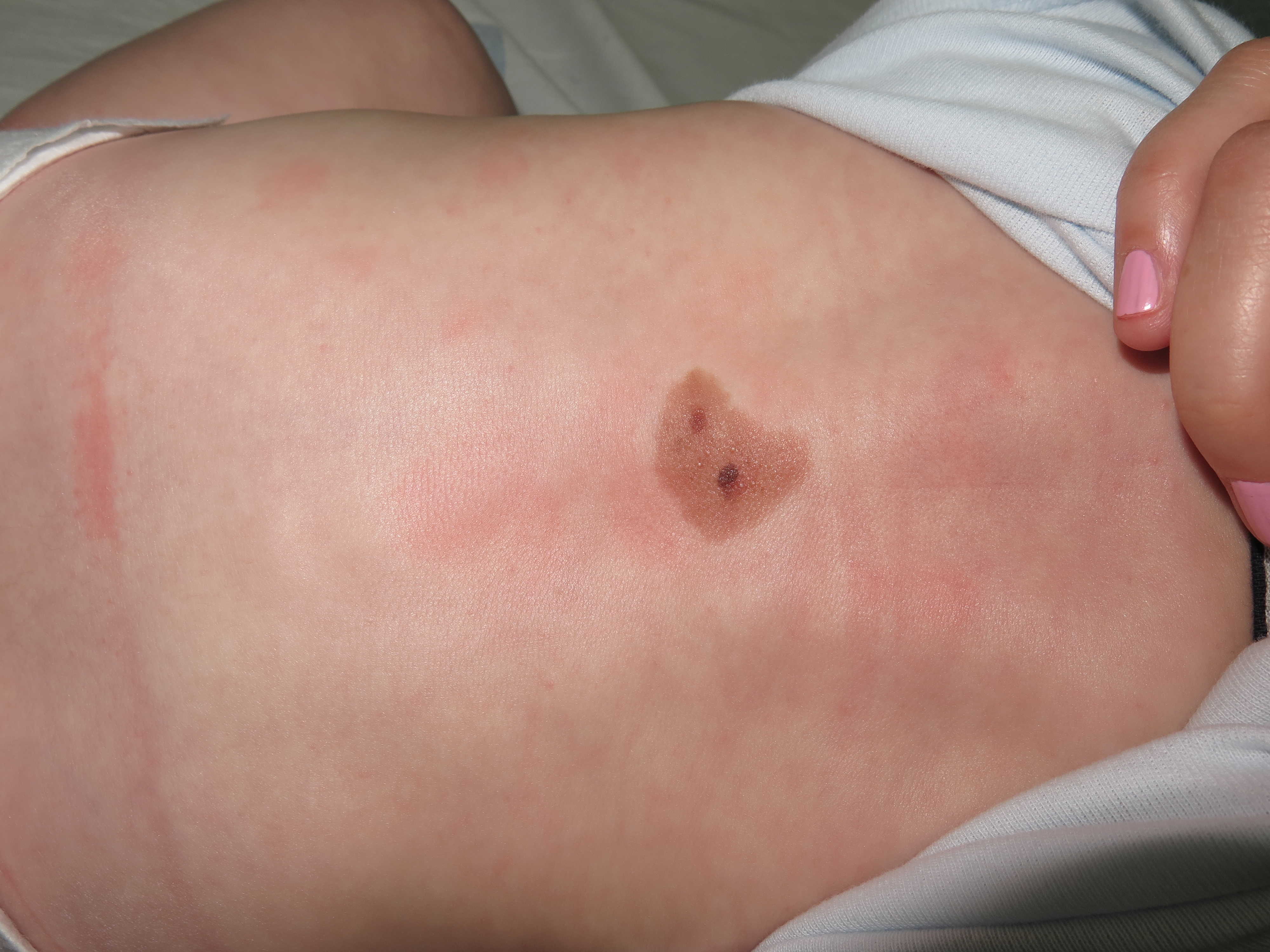
A congenital melanocytic nevus (CMN) is a benign skin lesion caused by nevomelanocyte proliferation, present at birth or appearing within the first few weeks of life (see Image. Medium-Sized Congenital Nevus on Infant's Back).[1] These lesions may also be called giant hairy nevi, conveying the frequent clinical presence of excess hair growth. CMNs are categorized as small, medium, large, or giant based on the lesion's projected maximal diameter in adulthood.[2]
Small CMNs measure less than 1.5 cm in diameter, medium lesions range from 1.5 to 19.9 cm, and large or giant lesions are 20 cm and larger.[3] The scaling factor used to predict adult size is based on anatomical location. A CMN located on the head is predicted to grow by a factor of 1.7, on the lower limb by 3.3, and upper limb and torso by 2.8.[4] The rationale for classification based on size is that larger lesions have a higher risk of melanoma, cosmetic implications, more challenging surgical excision considerations, and higher rates of associated symptoms.[5]
Etiology
CMNs develop in utero from the fifth to the 24th week of gestation due to localized genetic abnormalities that result in the over-proliferation of melanocytes.[6] Melanoblasts, melanocyte precursors, begin migrating from the neural crest to the epidermis between the eighth and tenth gestational weeks.[7] A common CMN mutation is on the NRAS gene, hypothesized to lead to the overproliferation of melanocyte lineage cells and, thus, the CMN formation.[8] Dysfunction of the cytokine hepatocyte growth factor, which regulates melanocyte migration and proliferation, has also been implicated in this condition.[9] Although some evidence exists of familial etiology, most lesions are presumptively caused by sporadic mutations. [7]
Epidemiology
Although the estimated prevalence varies between studies, smaller CMNs are considered relatively common lesions, while large CMNs are rare. The estimated incidence of small CMNs is 1 in 100 newborns, medium CMNs 1 in 1000, and large or giant CMNs between 1 in 20,000 to 1 in 500,000 newborns.[4][6][10] The reported incidence of CMNs is higher in females than males, with a ratio of 3:2.[7]
Histopathology
CMNs are typically characterized by nevomelanocytes or nevus cells in the epidermis as well-ordered clusters and in the dermis as cords, sheets, or nests. Compared to acquired nevi, CMNs frequently extend deeper into the dermis or subcutaneous fat layer.[11] The nevus cells cluster around dermal appendages such as the pilar apparatus, neurovascular structures, and sebaceous glands.[6][11] Nevus cells may also extend as cords of cells between bundles of collagen within the reticular dermis.[12]
History and Physical
CMNs are usually noted at birth or in the neonatal period. CMNs of small-to-medium size normally present as well-defined round or ovoid lesions with a smooth surface and uniform brown color, while larger CMNs may present with an irregular border, different pigmentations, and rugous or nodular surface textures.[4][13] Satellite nevi are also more common in larger CMNs than smaller ones, occurring in approximately 80% of giant CMNs (see Image. Giant Congenital Melanocytic Nevus in Newborn).[12][14]
CMNs may evolve with changes in pigmentation, the development of dermal nodules, and the development of hair in some lesions. Pigmented hair growth or hypertrichosis has an incidence of 75%.[7] Nodule formation is common in larger lesions and usually represents a benign proliferation of melanocytes. Although usually asymptomatic, larger lesions may have clinical features of xerosis, ulceration, pruritis, or skin erosion.[15] Furthermore, the unsightly aesthetic appearance can cause significant psychosocial impacts on children and parents alike.[7]
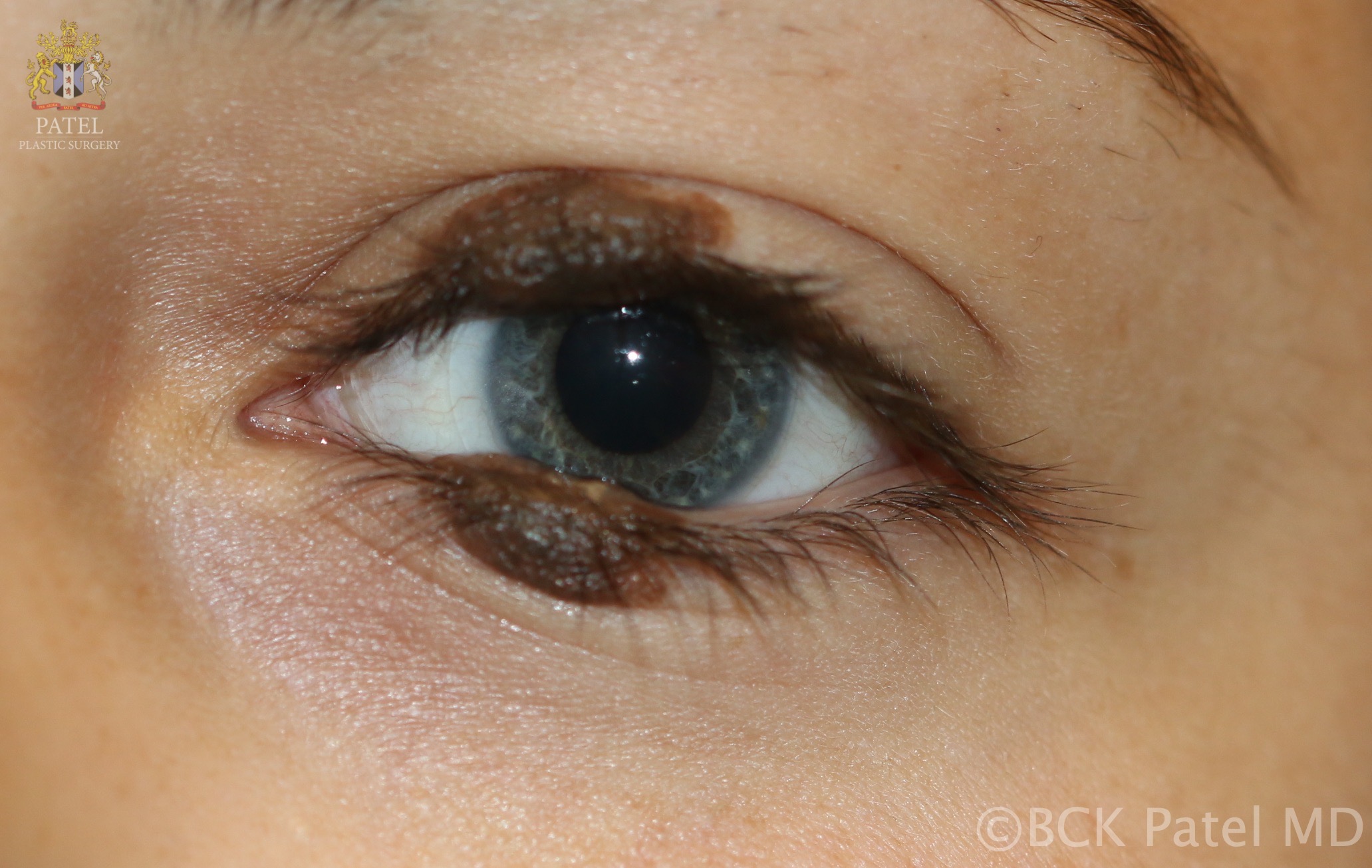
A particular type of nevus is the "divided nevus." These are paired nevi that may occur on the penis, fingers, or eyelids. They are sometimes called "kissing nevi," which were first described by Fuchs in 1919 (see Image. Congenital Melanocytes Nevus, "Kissing Nevus" Type). These nevi are a single lesion up to the 24th week of gestation. When the eyelids separate after the 24th week of gestation, the nevi divide. Although these nevi are generally present at birth, they can also present at a later age. Associated problems with eyelid kissing nevi are epiphora, eyelid malposition (ptosis or ectropion), or amblyopia.[16]
Evaluation
CMNs are usually diagnosed by their clinical appearance. Examination with dermatoscopy or biopsy for histopathological analysis may be used in cases of diagnostic doubt. Dermatoscopy most commonly reveals globular pigmentation patterns, while structureless, reticular, or mixed patterns may occur.[7] Results from one study defined structures that differentiate CMNs from acquired nevi as the presence of small globules, vessels, follicles, and target networks.[17] Tissue biopsy may rule out malignant differential diagnoses through histopathological assessment.
Treatment / Management
Treatment options may be divided into surgical and nonsurgical. Surgical options include tangential excision, curettage, en bloc or serial excision and direct closure, and excision with reconstruction using tissue expansion, skin graft, or flap.[18][19] Nonsurgical treatments include dermal abrasion, chemical peels, cryotherapy, electrosurgery, and laser ablation.[6] These treatments may be considered to reduce pigmentation and improve the cosmetic appearance of the nevus without fully removing nevi cells.
Historically, the rationale for treatments for CMNs has been influenced by improving their aesthetic appearance and reducing the risk of malignant change. However, excision surgery to reduce malignancy risk remains controversial. Advocates of surgery argue that the incidence of melanoma is lower in those who have undergone excision than those with observation alone.[20] Arguments against surgery include the low absolute incidence of melanoma in CMN balanced with surgical risks. Excision does not impact the risk of extracutaneous melanoma occurrence. Extracutaneous melanoma accounts for a significant proportion of new melanomas in this population.[3] As a result, many centers do not offer surgical excision based on risk reduction alone. Instead, they offer surveillance unless there are aesthetic or function indications for surgery. Further randomized studies are warranted.
Differential Diagnosis
Congenital melanocytic nevus closely resembles and must be differentiated from the following:
- Acquired melanocytic nevus
- Congenital blue nevus
- Lentigo
- Mongolian spots
- Nevus spilus
- Becker nevus
- Café-au-lait spots
- Pigmented epidermal nevus
Prognosis
Small and medium CMNs pose fewer risks regarding malignant transformation and neurocutaneous melanosis complications. These lesions generally have a good prognosis for patient survival. When melanomas arise in large or giant CMN, the prognosis is poor. The reported 5-year survival in 35 patients with giant CMNs with diagnosed melanomas was 34.3%.[5] This poor prognosis is hypothesized to be due to delayed clinical detection due to the deeper origin of cutaneous tumors, as well as de novo extracutaneous melanoma formation.[6] The deeper cutaneous origin of the melanomas may also cause accelerated lymphatic and metastatic spread, with one report indicating 24% of melanomas in patients with giant CMN had metastases at the time of diagnosis.[21] For patients with symptomatic neurocutaneous melanosis, the prognosis is also unfavorable. Results from one study reported that 50% of patients died within 3 years of symptomatic onset, with no curative treatments yet identified.[22]
Complications
Complications are more common in patients with larger CMNs, ranging from localized cutaneous irritation to malignant melanoma transformation.[12] CMNs may develop symptoms of cutaneous irritation, such as ulceration and pruritus, as well as distortion in the structure and function of specific anatomical sites. A psychological burden to children and parents based on aesthetic appearance is a frequent sequela in such cases. With larger lesions, systemic involvement may comprise limb abnormalities and scoliosis.[23] The most severe complications include malignant melanoma and neurocutaneous melanosis.
Malignant melanomas may develop in any patient with CMN, although the risk is higher in patients with large lesions greater than 20 cm in size or with multiple CMNs.[23] Malignant transformation may occur cutaneously or extracutaneously, including the central nervous system, retroperitoneum, and the mucosa of the gastrointestinal tract. The reported absolute risk of melanoma with CMNs varies between study results. Patients with small to medium CMNs have an absolute risk of 0 to 4.9%, while in patients with large CMNs, the absolute risk is 1.25 to 10.00%.[4][24][25] Due to the higher risk, patients with large CMNs require long-term monitoring for cutaneous and extracutaneous malignancy features.
Meningeal melanocytosis is associated with giant or multiple CMNs, characterized by benign or malignant proliferation of melanocytes in the central nervous system.[6] Neurocutaneous melanosis may be asymptomatic or symptomatic regardless of malignant transformation. Although this condition has been estimated to affect 5% to 10% of patients with giant CMN, most are asymptomatic.[4] The true incidence has not been determined. In neurocutaneous melanosis, the presence of melanocyte proliferation or hemorrhages may cause clinical features of raised intracranial pressure, such as seizures, focal cranial nerve palsies, or hydrocephalus.[12] These diagnoses are made through magnetic resonance imaging radiology and with medical and neurosurgical management within the interprofessional setting.
Deterrence and Patient Education
Healthcare professionals must be knowledgeable about the appearance of CMNs to identify these lesions at birth or soon afterward. Importantly, ensure appropriate diagnosis and referral to dermatology. Due to the higher risk of severe complications, patients with large CMNs require long-term monitoring for features of both cutaneous and extracutaneous malignancies.
Enhancing Healthcare Team Outcomes
CMN is a benign skin lesion present at birth or shortly after, categorized as small (<1.5 cm), medium (1.5–19.9 cm), or large/giant (≥20 cm). Larger CMNs carry higher melanoma risks (1.25–10%) and pose cosmetic and surgical challenges. Symptoms may include pigmentation changes, hypertrichosis, and nodule formation, with psychosocial impacts often significant. Diagnosis involves clinical evaluation, dermatoscopy, or biopsy. Treatment options include surgical excision or nonsurgical methods to improve aesthetics; however, the effectiveness of excision in reducing melanoma risk remains debated. Patients with large CMNs require long-term monitoring for malignancy.
An interprofessional team approach is strongly advocated in caring for children with this condition, particularly for larger lesions. Pediatricians and family medicine clinicians should be able to diagnose CMNs and refer the patient to dermatology. Regular assessments involve dermatology examinations, with input from plastic surgery as required. Monitoring for progression with serial photographs and malignant progression with input from pathologists for tissue diagnosis as required is also important.[12] Pediatric psychologists and psychiatrists also play an integral role in managing the psychosocial burden.[23] Pediatric nursing and allied health professionals also play a pivotal role in management. Communication and coordinated care between interprofessional team members is important to ensure good patient outcomes.