Continuing Education Activity
Basic flap design is a fundamental aspect of reconstructive surgery, involving the creation of tissue flaps to restore form and function in areas of tissue loss or defect. Flap design aims to provide adequate blood supply to the transferred tissue while ensuring optimal aesthetic and functional outcomes. Key principles include selecting the appropriate flap type based on the defect's location and size, considering the surrounding tissues' vascularity, and planning incisions to preserve neurovascular structures and minimize donor site morbidity. Common flap designs include local, regional, and free flaps, each offering distinct advantages and limitations depending on the specific clinical scenario. Attention to detail in flap design is essential for successful outcomes, and tissue laxity, scar orientation, and aesthetic subunits must be carefully considered to achieve optimal wound healing and cosmesis.
Clinicians participating in this course on basic flap design can expect to gain a comprehensive understanding of the principles and techniques involved in flap design for reconstructive surgery. Participants will learn to assess patient anatomy and pathology to determine the most appropriate flap design for specific clinical scenarios. Emphasis will be placed on optimizing flap vascularity, minimizing complications, and achieving favorable aesthetic and functional outcomes. Overall, this course equips clinicians with the knowledge and skills to effectively plan and execute flap procedures, ultimately improving patient care and outcomes in reconstructive surgery.
Objectives:
Identify the anatomical features of tissue defects requiring flap reconstruction.
Differentiate between flap types based on tissue composition, vascularity, and donor site location.
Assess flap viability intraoperatively and postoperatively to identify signs of ischemia or necrosis.
Collaborate with multidisciplinary teams, including surgeons, nurses, and anesthesiologists, to optimize perioperative care.
Introduction
A fundamental skill of reconstructive surgery is flap design and transfer to close tissue defects that cannot be sutured primarily. Given the diverse spectrum of tissue defects encountered in patients, ranging from small, skin-only defects to extensive, multi-tissue-type defects, and the various underlying causes, the skill set required for flap transfer must be adaptable and continually evolving as new techniques are described. Flaps are often employed for wound closure when substantial tissue loss occurs during trauma or as a result of oncologic resection. Classic examples include traumatic scalp avulsions requiring closure with latissimus dorsi free flaps and hemiglossectomies requiring reconstruction with radial forearm free flaps.[1][2]
Understanding the anatomy and physiology of the defect and potential donor sites is critical to successful flap transfer, as is mastery of atraumatic soft tissue surgical technique. Ultimately, flap design and transfer represent the clinical intersection of the science of medicine and the art of surgery, which renders these procedures either immensely rewarding or singularly frustrating, depending on the outcome.[3]
Anatomy and Physiology
Flaps may be classified in several ways, depending on blood supply, tissue type, and the distance of the harvest site from the tissue defect. Each scheme has its own set of anatomical and physiological implications.
Blood Supply
Flaps are generally divided into "random" and "axial" categories when classified according to blood supply.
- Axial flaps
- These flaps derive their blood supply from a named artery that courses longitudinally within the flap, with venous drainage primarily through corresponding veins. To improve survivability, an axial flap will typically include tissue from a single angiosome only, a region of tissue supplied predominantly by a single named blood vessel. In some cases, a flap may consist of tissue from more than 1 angiosome despite having only one named artery within the vascular pedicle (the flap's base containing the arterial supply and venous drainage). The classic example of a flap that includes multiple angiosomes is the deltopectoral flap, based on intercostal perforating arteries branching off the internal mammary artery.[4]
- Random flaps
- Random pattern blood supply flaps rely on perfusion from the subdermal vascular plexus, a low-pressure system vulnerable to compromise under excessive twisting or stretching. Due to the tenuous blood supply, designing these flaps with a length-width ratio not exceeding 3:1 is advisable.[5]
Tissue Type
Flaps may also be classified according to the tissue type involved, with the tissue types incorporated in the flap typically corresponding to the tissue types missing from the defect. Most smaller flaps will be cutaneous, but mucosal, bony, muscle, and fascial flaps are common.
Larger defects often require "composite" reconstruction (multiple tissue types together), potentially including fasciocutaneous, myocutaneous, and osteocutaneous flap transfers. Classic examples of composite tissue transfer include the radial forearm free flap, the pectoralis major flap, and the fibula free flap. Human composite tissue allograft transfer, such as hand or face transplant, also falls into this category.[6]
Distance
Flaps are often categorized based on the distance between the donor site and the tissue defect.
- Distant
- "Free" or "microvascular" flaps are harvested from distant sites, removed entirely from the body, and then transplanted into defects, where their vascular supplies are reconnected through microsurgery. These are frequently composite flaps with more than 1 tissue type and are always axial in design.
- Common examples include the anterolateral thigh flap, the iliac crest flap, and the gracilis flap.
- Regional
- "Regional" flaps are harvested from the same region as the defect, such as the same limb or elsewhere in the head and neck. Unlike free flaps, regional flaps aren't entirely removed from the body and don't directly border the defect. Typically axial in design, these flaps may or may not contain composite tissue.[7]
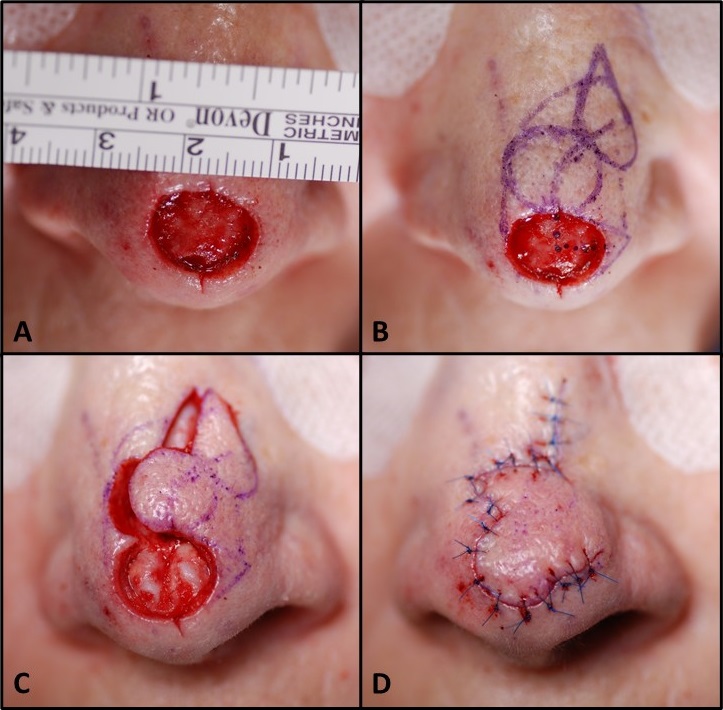
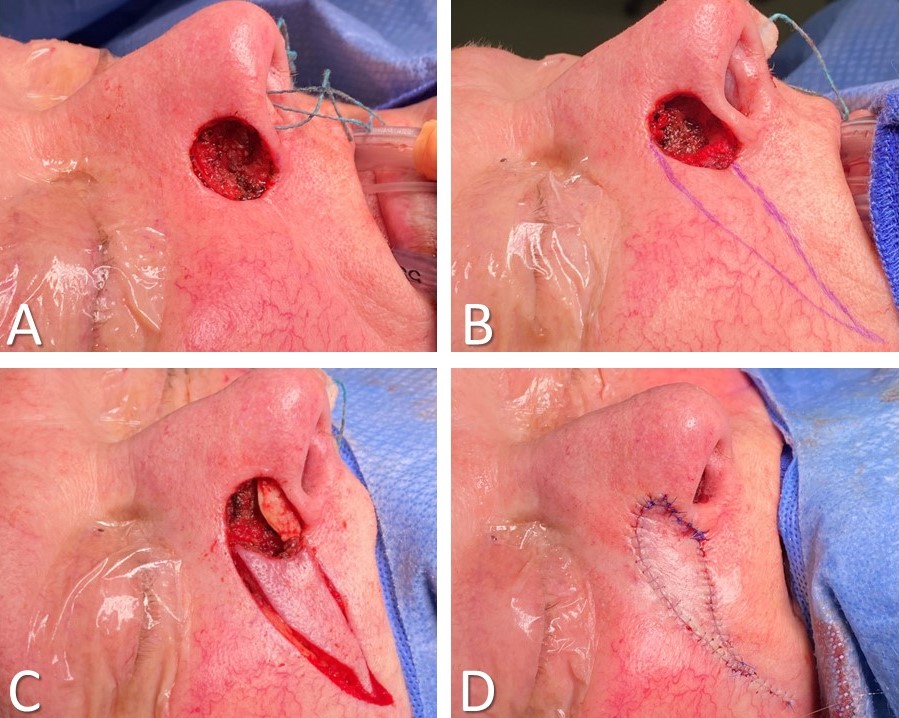
- Classic examples of regional flaps include the pedicled gracilis flap, the temporoparietal fascia flap, and the paramedian forehead flap (see Image. Paramedian Forehead Flap for Nasal Reconstruction).
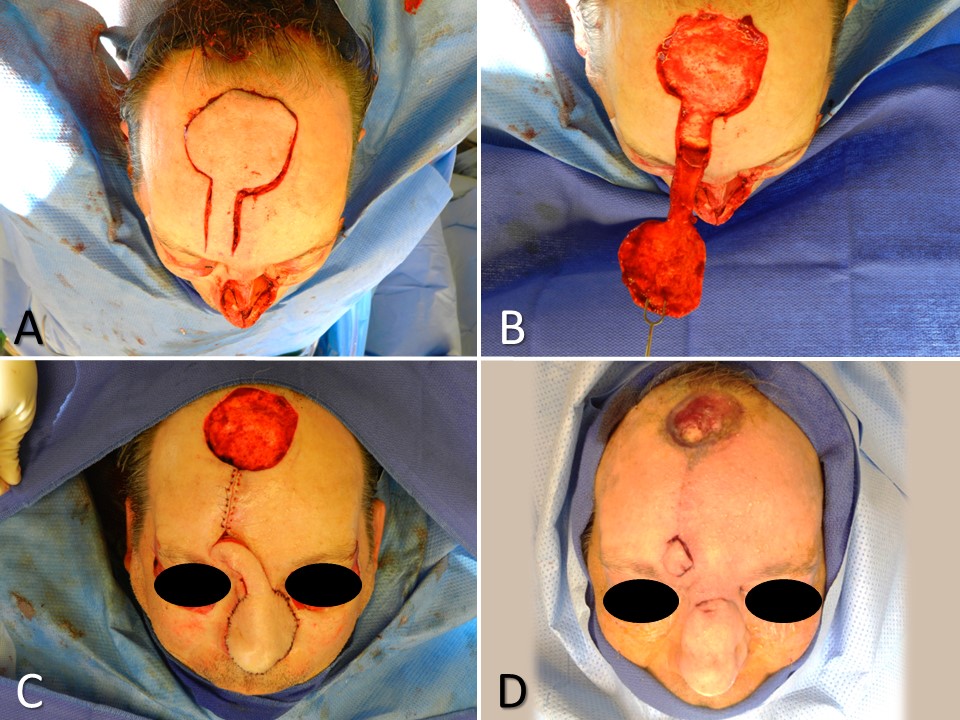
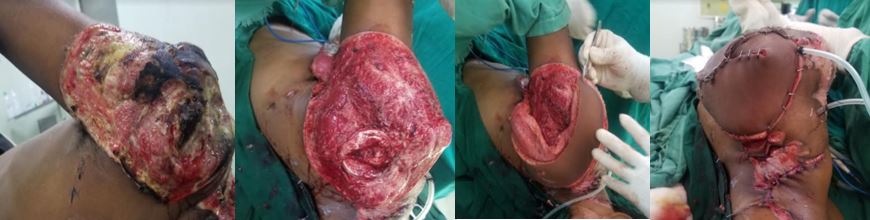
- Several flaps, particularly muscular ones such as the trapezius and latissimus dorsi, can be transferred as free or regional flaps. This choice hinges on the configuration and location of the defect relative to the flap harvest site (see Image. Latissimus Dorsi Flaps). Free flaps are typically favored for closing larger defects (around 30 cm² or more), while regional flaps can effectively cover large and small defects.[7]
- Local
- "Local" flaps originate from donor sites directly adjacent to the tissue defect, incorporating the donor site into the closure process. When utilizing local flaps, it's crucial to consider the resulting scar configuration and its optimal placement within aesthetic subunit boundaries, such as the nasolabial fold or the hairline or along relaxed skin tension lines. Typically relying on random pattern blood supplies, local flaps are primarily used for smaller defects and consist predominantly of cutaneous or mucosal tissue. They are also categorized based on the type of movement required to close the defect.
- The 4 types of movement are advancement, rotation, transposition (in which the flap and other normal tissue adjacent to the defect trade places), and interpolation (in which the flap traverses either over or under normal tissue to reach the defect).
- In practice, interpolation is more frequently used in regional flaps than local flaps, and rotation and advancement are employed to close many defects.
- Some categorize rotational, transpositional, and interpolated flaps together as "pivotal" flaps.
Indications
Flap transfer is indicated when a tissue defect cannot be closed primarily, and it would be inappropriate to allow the defect to heal by secondary intention or with a skin graft.
Contraindications
An absolute contraindication to flap transfer for wound closure arises when the defect can be safely, aesthetically, and effectively closed either primarily or with a skin graft.
Relative contraindications include an active infection in the wound or residual malignancy after oncologic resection. Flap transfer may proceed once the infection has resolved or margins have been cleared. Positive oncological margins may not necessarily contraindicate flap transfer in palliative care cases. Notably, tobacco users are at high risk for vascular compromise of flaps, making current smoking a relative contraindication.[8][9]
Equipment
Equipment requirements vary greatly depending on the type of flap being transferred.
Local flaps may require no more than fine soft-tissue instruments and local anesthesia. In contrast, larger regional and free flaps may require extensive instrumentation, including soft tissue sets, bone saws, an operating microscope, microvascular tools, and vasoactive medications.
For the average in-office local flap case, eg, Mohs defect closure, the following equipment may be utilized:
- Scalpel (#15 Bard-Parker blade or #6700 Beaver blade)
- Forceps (Adson-Brown or 0.5 mm Castroviejo)
- Needle holder (Halsey or Castroviejo)
- Dissecting scissors (Iris or Kaye blepharoplasty)
- Suture scissors
- Electrocautery (monopolar or bipolar)
- Sutures (5-0/6-0 polypropylene, 4-0/5-0 poliglecaprone)
- Adhesive tape strips
- Skin marker
- Local anesthetic (1% lidocaine with 1:100,000 epinephrine)
- Antiseptic scrub solution (povidone-iodine or isopropanol)
Personnel
Personnel requirements vary according to the demands of the case, but at minimum, a surgeon and nurse will be needed. An anesthesia provider, a surgical technician, and a surgical first assistant are required for larger cases. Postoperatively, nursing and physical therapy may be necessary as well, particularly if the defect or donor site is located in a limb.
Preparation
Surgeons must carefully evaluate potential patients and their medical histories to develop a tailored treatment plan that addresses each patient's specific needs and risk factors, ultimately optimizing outcomes and minimizing complications.
Preoperative considerations include:
- Smoking status
- Atherosclerosis
- Peripheral vascular disease
- Steroid use
- Diabetes
- Previous surgeries at potential flap harvest sites
- Extent of the defect
- Patient age and skin condition
- Defect location [3]
A preoperative angiogram can help assess circulation and anatomy before a free flap or regional flap transfer. Since larger defects typically necessitate more intricate reconstruction, which prolongs surgical duration, it is imperative to ensure that patients undergo thorough medical preoperative evaluations.
When performing a flap transfer, careful planning that addresses technical aspects of the procedure and the patient's overall health and functional and aesthetic goals can result in satisfactory outcomes in most cases.[10]
Technique or Treatment
The Reconstructive Ladder
When determining the appropriate flap to use for the closure of a defect, surgeons often think through a construct known as the "reconstructive ladder," which enumerates the options from the least invasive (healing by secondary intention) to the most complex (transplant). The reconstructive ladder also includes options not strictly classified as flaps but are nevertheless important for the closure of defects. The ladder is as follows:
- Healing by secondary intention
- Primary closure
- Delayed primary closure
- Skin and composite graft transfer (full thickness or split thickness)
- Local flap transfer (may include tissue expansion)
- Regional flap transfer
- Free flap transfer
- Composite tissue allograft (transplant)
Free Flap Technique
Free tissue transfer techniques in reconstructive surgery are diverse and contingent upon various factors, including flap type and surgeon preference. Key considerations involve ensuring the flap comprises the appropriate tissue types in sufficient quantities and that the vascular pedicle is of adequate length to reach the recipient vessels for perfusion. Typically, only a single angiosome is harvested with a free flap, necessitating thorough perfusion assessment to guarantee adequate tissue viability.[11] Techniques vary, with some involving identifying and dissecting the vascular pedicle before flap elevation, as seen in the gracilis flap, while others, like the radial forearm flap, begin with flap elevation followed by vascular dissection.[12][13]
Following flap harvest, meticulous monitoring of ischemia time is initiated using a clock to track the duration of tissue ischemia. Prolonged ischemia can lead to the "no-reflow" phenomenon, hindering flap reperfusion after microsurgical anastomosis due to intravascular clotting. Flap inset precedes microsurgical anastomosis to ensure correct vascular pedicle geometry, minimizing the risk of flap distortion. Microsurgical anastomosis necessitates specialized equipment, including an operating microscope, microsurgical instruments, and fine sutures, typically ranging from 8-0 to 10-0. Additionally, venous couplers are often employed to facilitate precise vascular connections. In buried flaps, a monitor paddle may be brought to the skin to facilitate postoperative monitoring, or an implantable Doppler probe may be used to evaluate blood flow in real-time.
Maintaining correct pedicle geometry is crucial to ensure optimal flap perfusion and prevent venous outflow obstruction, a potential complication. The initial 3 postoperative days pose the highest risk of clot formation in the freshly sewn blood vessels, as it takes around 72 hours for vessel endothelia to heal over sutures, heightening the risk of flap failure. Regular flap checks during this period are essential to detect vascular issues promptly. As time progresses, the risk diminishes until neovascularization occurs, roughly 2 to 3 weeks postsurgery.[14]
Since free flaps are often sizable, secondary donor site reconstruction is common, usually achieved with a split-thickness skin graft.
Regional Flap Technique
Regional tissue transfer, akin to free tissue transfer, offers versatility in surgical options, with several flaps adaptable for both regional and free transfer, including but not limited to the radial forearm flap, gracilis flap, trapezius flap, latissimus dorsi flap, temporoparietal fascia flap, and strap muscle flap (see Image. Temporoparietal Fascia Flap). While regional flaps and free flaps share considerations regarding tissue type and volume, their primary distinction lies in vascular continuity; regional flaps maintain a consistent blood supply and are never detached from the body, typically classified as interpolated flaps.
While regional flap vascular pedicles are less tenuous than free flaps in the initial 72 hours postoperatively, they are susceptible to stretching and twisting. Regional flaps may necessitate significant manipulation of their vascular pedicles due to donor and recipient site configurations. Classic examples include the paramedian forehead flap, pectoralis major myocutaneous flap, and supraclavicular artery island flap.[15][16][17]
The advantages of regional flaps over free flaps encompass reliability, obviating the need for microsurgical equipment, reduced operative time (enhancing safety for patients with multiple comorbidities), and the option to elevate and delay flap transfer preoperatively for approximately 2 weeks, thereby improving survival rates.[18] However, as with free flaps, the harvest site may require secondary reconstruction with skin grafting.
Local Flap Technique
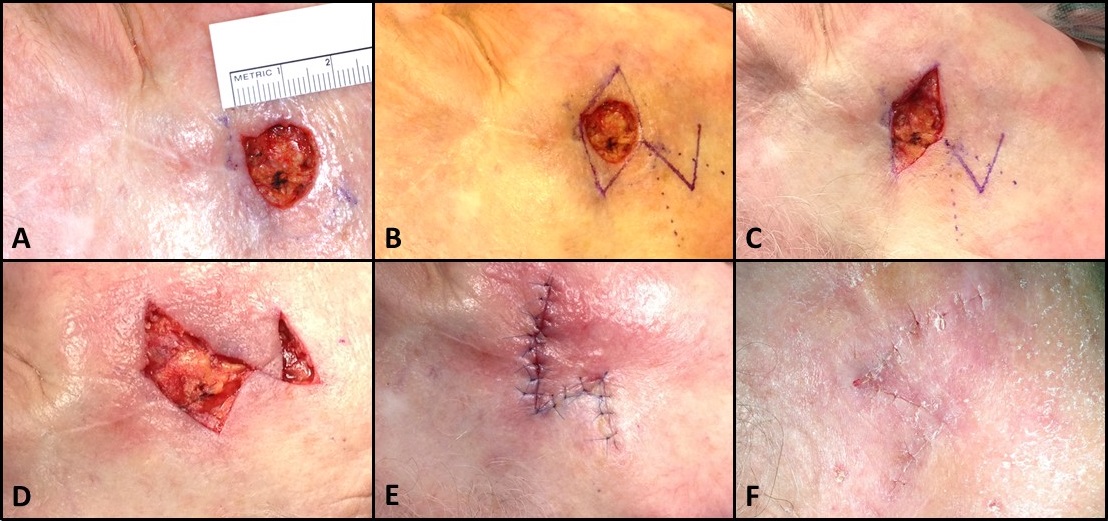
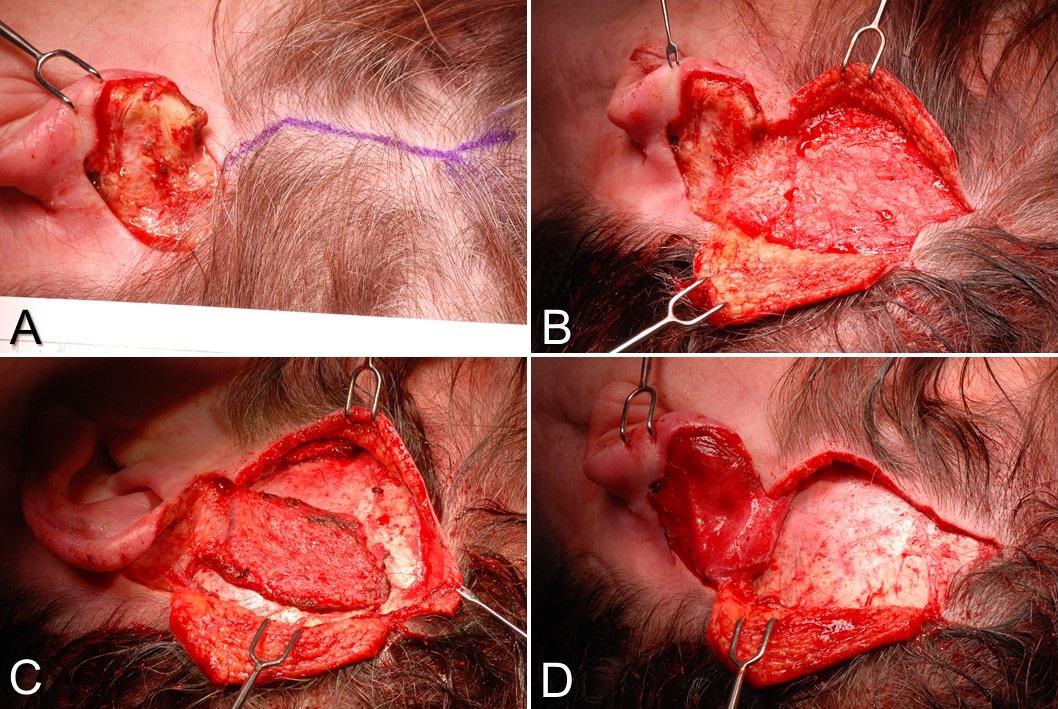
Local flaps, by definition, consist of tissue that lies adjacent to the defect; to reach their destinations, they may be advanced, rotated, transposed, interpolated, or any combination thereof.[5][19][20][21] Classic examples include O-to-H flaps, O-to-Z flaps, rhombic and bilobed flaps, and melolabial flaps (see Image. Rhombic Flap for Mohs Defect of the Right Cheek). Unlike regional and free flaps, local flaps tend to have random pattern blood supplies, meaning there is no vascular pedicle but rather that the subdermal plexus supplies oxygenated blood and provides venous drainage. The base of the flap, which connects the flap to the surrounding tissue, should be handled with care to prevent stretching or twisting in order to maintain optimal perfusion and ensure flap viability.
Given their reliance on microvasculature for perfusion, meticulous elevation in the correct plane is crucial, with electrocautery use minimized. The appropriate elevation plane varies based on the anatomical region. For instance, subdermal elevation is standard, while the scalp may necessitate a subgaleal plane and the nose a supraperichondrial plane (see Image. Bilobed Flap, Zitelli Modification).
In general, local flaps should adhere to a length-width ratio where the length does not exceed 3 times the width of the base. This guideline helps prevent vascular insufficiency at the distal tip, especially if the flap base requires twisting during inset.[5] Wide undermining of up to 2 to 4 cm is initially undertaken to close the primary tissue defect. This technique is executed cautiously to avoid disturbing delicate structures in the vicinity, such as the eyelids or nostrils while determining the direction in which tissues can most easily migrate into the defect.[22]
An important exception to this undermining suggestion is V-to-Y advancement flaps, as excessive undermining can lead to vascular insufficiency. V-to-Y island advancement flaps require approximately one-third of their surface area to remain connected to the subdermal fat to ensure survival. Undermining should be completed for most other cases, and the flap should be transferred. Subsequently, tacking sutures should be placed around the flap and to close the secondary defect (where the flap was harvested). Burow triangles are then excised to address standing cutaneous deformities (see Image. V-to-Y Island Advancement Flap). A Burow triangle is a wedge of skin removed from the base of a flap to flatten a standing cutaneous deformity that arises when a flap is rotated or advanced, particularly when a flap is rotated more than 90 degrees. These excisions are strategically placed to minimize scarring outside relaxed skin tension lines or aesthetic subunit boundaries.[19]
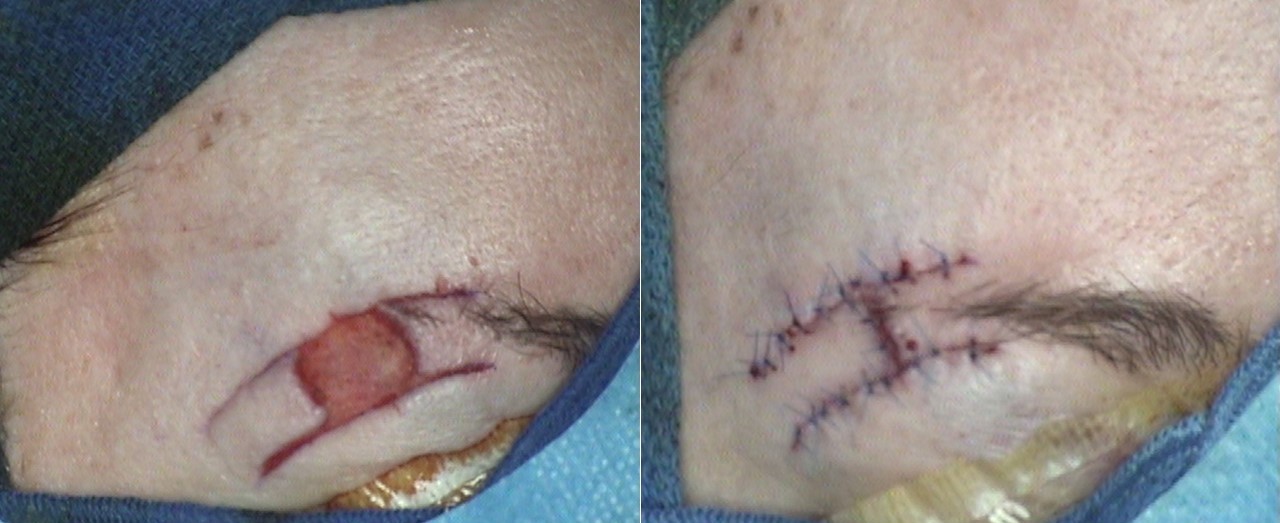
Relaxed skin tension lines and aesthetic subunit boundaries are crucial in local flap design. Although defect size and location may not always be predictable, careful planning ensures that the flap transfer does not distort nearby structures such as the lips, ears, or hairline. Additionally, positioning the flap to align with aesthetic subunit boundaries and relaxed skin tension lines helps minimize visible scarring and achieve optimal cosmetic outcomes (see Image. O-to-H Bilateral Advancement Flaps to Close a Brow Defect).
Complications
Complications arising from flap transfer can be categorized into donor site problems and flap problems, further divided into acute and chronic complications.
Donor Site Complications
Donor site complications encompass various issues such as bleeding, infection, scarring, and functional impairment, which can range from gait disturbances to hand contractures in the long term.
Specific complications are unique to each donor site; for instance, harvesting an iliac crest free flap may lead to an abdominal hernia, an osteocutaneous radial forearm free flap may result in pathological wrist fractures, while a peroneal nerve injury during a fibula free flap harvest can lead to foot drop.[23][24]
Flap Complications
Complications associated with the flap itself predominantly involve acute compromise of the flap's blood supply and subsequent failure of the reconstruction. Generally, the larger the defect, the more significant the consequences of flap death.[25]
When flaps undergo necrosis, vascular compromise typically underlies the issue, with obstruction of venous outflow being the primary cause.[14] In larger free or regional flaps, clot formation in the main draining vein often leads to compromised blood flow. Conversely, in local flaps, the venous aspect of the subdermal plexus may be affected. Excessive tension or torsion can also impact the subdermal plexus, rendering the flap susceptible to inadequate blood supply. In cases where tissue width or quality is insufficient to support proper blood flow, partial or complete flap necrosis may occur.
Vascular insufficiency in flaps can also result from compression due to hematoma formation, necessitating prompt evacuation if identified. In cases where vascular problems arise in free flaps, revision of the microvascular anastomosis can improve blood flow. However, revising the inset to alleviate tension or torsion on the pedicle may be necessary for regional or local flaps. Venous obstruction in any flap can be addressed temporarily with medicinal leeches to aid in reestablishing venous outflow. When using leeches, prophylactic administration of a fluoroquinolone antibiotic, such as levofloxacin, is crucial to prevent infection with Aeromonas hydrophila, a gram-negative bacillus commonly associated with leeches.[26]
Several factors can contribute to early flap failure, such as infection, inadequate blood pressure, nutrition status, and patient compliance.[27] Generally, if a flap shows no signs of venous obstruction (ie, duskiness or firmness) or arterial insufficiency (ie, pallor and coolness) during the first week after surgery, it is likely to remain viable. However, chronic complications may arise, affecting both function and aesthetics. These include scarring, contracture, color or texture discrepancies, abnormal hair growth or loss, numbness, and persistent pain. Therefore, close monitoring and prompt intervention are crucial to address issues and ensure optimal outcomes for patients undergoing flap procedures.
Clinical Significance
Flap transfer has indeed been fundamental to reconstructive surgery for over 3000 years, as documented in the Sushruta Samhita, which describes forehead flap utilization for nasal defect closure.[28] While the techniques have evolved significantly over the intervening millennia, the concept of reconstructing the human body using tissue that appears and performs similarly to the missing tissue remains the same.
A well-designed flap should use the minimal amount of tissue required, cause the least amount of donor site morbidity, and maximize the return of form and function to the tissue defect while simultaneously optimizing the flap's survivability to prevent the need for revision surgery in the future. The astute reconstructive surgeon will maintain familiarity with a wide range of flap transfer options to suit a wide range of defect tissues and sizes to enhance patient outcomes.
Enhancing Healthcare Team Outcomes
Effective patient care regarding flap procedures requires a coordinated effort from a multidisciplinary team comprising physicians, advanced practitioners, nurses, pharmacists, physical therapists, and other health professionals. Physicians play a crucial role in assessing patients' suitability for flap surgery, selecting appropriate surgical techniques, and managing postoperative complications. Advanced practitioners, such as physician assistants and nurse practitioners, provide comprehensive preoperative evaluations, assist in surgical procedures, and facilitate patient education and follow-up care. Nurses are integral in monitoring patients before, during, and after surgery, assessing flap viability, managing wound care, and providing patient education on postoperative care and preventing complications. Pharmacists collaborate with the team to ensure appropriate medication management, including prophylactic antibiotics and pain management regimens tailored to individual patient needs. Physical therapists are essential in promoting optimal postoperative rehabilitation, facilitating early mobilization, and implementing strategies to prevent postoperative complications such as contractures and stiffness. Interprofessional communication and collaboration among team members are vital to ensure seamless care transitions, effective management of complications, and patient-centered decision-making throughout the perioperative period, ultimately enhancing patient safety, outcomes, and overall team performance.