[1]
Eisma JH, Dulle JE, Fort PE. Current knowledge on diabetic retinopathy from human donor tissues. World journal of diabetes. 2015 Mar 15:6(2):312-20. doi: 10.4239/wjd.v6.i2.312. Epub
[PubMed PMID: 25789112]
[2]
Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic Retinopathy. Primary care. 2015 Sep:42(3):451-64. doi: 10.1016/j.pop.2015.05.005. Epub
[PubMed PMID: 26319349]
[3]
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England journal of medicine. 1993 Sep 30:329(14):977-86
[PubMed PMID: 8366922]
[4]
. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England). 1998 Sep 12:352(9131):837-53
[PubMed PMID: 9742976]
[5]
Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World journal of diabetes. 2015 Feb 15:6(1):92-108. doi: 10.4239/wjd.v6.i1.92. Epub
[PubMed PMID: 25685281]
[6]
Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular Complications of Diabetes and Therapeutic Approaches. BioMed research international. 2016:2016():3801570. doi: 10.1155/2016/3801570. Epub 2016 Mar 28
[PubMed PMID: 27119078]
[7]
Khan A, Petropoulos IN, Ponirakis G, Malik RA. Visual complications in diabetes mellitus: beyond retinopathy. Diabetic medicine : a journal of the British Diabetic Association. 2017 Apr:34(4):478-484. doi: 10.1111/dme.13296. Epub 2016 Dec 20
[PubMed PMID: 27917530]
[8]
Nittala MG, Keane PA, Zhang K, Sadda SR. Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina (Philadelphia, Pa.). 2014 Aug:34(8):1594-9. doi: 10.1097/IAE.0000000000000117. Epub
[PubMed PMID: 24662751]
[9]
Porta M, Sjoelie AK, Chaturvedi N, Stevens L, Rottiers R, Veglio M, Fuller JH, EURODIAB Prospective Complications Study Group. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia. 2001 Dec:44(12):2203-9
[PubMed PMID: 11793022]
[10]
Wang Y, Lin Z, Zhai G, Ding XX, Wen L, Li D, Zou B, Feng KM, Liang YB, Xie C. Prevalence of and Risk Factors for Diabetic Retinopathy and Diabetic Macular Edema in Patients with Early- and Late-Onset Diabetes Mellitus. Ophthalmic research. 2022:65(3):293-299. doi: 10.1159/000508335. Epub 2020 Apr 30
[PubMed PMID: 32353847]
[11]
Schreur V, van Asten F, Ng H, Weeda J, Groenewoud JMM, Tack CJ, Hoyng CB, de Jong EK, Klaver CCW, Jeroen Klevering B. Risk factors for development and progression of diabetic retinopathy in Dutch patients with type 1 diabetes mellitus. Acta ophthalmologica. 2018 Aug:96(5):459-464. doi: 10.1111/aos.13815. Epub
[PubMed PMID: 30188024]
[12]
Wilkinson-Berka JL, Miller AG. Update on the treatment of diabetic retinopathy. TheScientificWorldJournal. 2008 Feb 6:8():98-120. doi: 10.1100/tsw.2008.25. Epub 2008 Feb 6
[PubMed PMID: 18264628]
[13]
Moutray T, Evans JR, Lois N, Armstrong DJ, Peto T, Azuara-Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. The Cochrane database of systematic reviews. 2018 Mar 15:3(3):CD012314. doi: 10.1002/14651858.CD012314.pub2. Epub 2018 Mar 15
[PubMed PMID: 29543992]
Level 1 (high-level) evidence
[14]
Gupta V, Arevalo JF. Surgical management of diabetic retinopathy. Middle East African journal of ophthalmology. 2013 Oct-Dec:20(4):283-92. doi: 10.4103/0974-9233.120003. Epub
[PubMed PMID: 24339677]
[15]
Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacological research. 2015 Sep:99():137-48. doi: 10.1016/j.phrs.2015.05.013. Epub 2015 Jun 6
[PubMed PMID: 26054568]
[16]
Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat(®). Progress in retinal and eye research. 2016 Sep:54():1-29. doi: 10.1016/j.preteyeres.2016.04.006. Epub 2016 Apr 19
[PubMed PMID: 27102270]
[17]
Kern TS, Antonetti DA, Smith LEH. Pathophysiology of Diabetic Retinopathy: Contribution and Limitations of Laboratory Research. Ophthalmic research. 2019:62(4):196-202. doi: 10.1159/000500026. Epub 2019 Jul 30
[PubMed PMID: 31362288]
[18]
Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. International journal of molecular sciences. 2018 Jun 20:19(6):. doi: 10.3390/ijms19061816. Epub 2018 Jun 20
[PubMed PMID: 29925789]
[19]
Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vision research. 2017 Oct:139():93-100. doi: 10.1016/j.visres.2017.03.013. Epub 2017 Sep 5
[PubMed PMID: 28866025]
[20]
Curtis TM, Hamilton R, Yong PH, McVicar CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S, Stitt AW. Müller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011 Mar:54(3):690-8. doi: 10.1007/s00125-010-1971-x. Epub 2010 Nov 30
[PubMed PMID: 21116609]
[21]
Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes HP, Bringmann A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006 Mar:55(3):633-9
[PubMed PMID: 16505225]
[22]
Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. Journal of diabetes research. 2013:2013():905058. doi: 10.1155/2013/905058. Epub 2013 Jun 12
[PubMed PMID: 23841106]
[23]
Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (London, England). 2002 May:16(3):242-60
[PubMed PMID: 12032713]
[24]
Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. Journal of cellular physiology. 2001 Aug:188(2):253-63
[PubMed PMID: 11424092]
[25]
Freyberger H, Bröcker M, Yakut H, Hammer J, Effert R, Schifferdecker E, Schatz H, Derwahl M. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2000:108(2):106-9
[PubMed PMID: 10826517]
[26]
Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M, Paoletti I, Barra A, Tucci M, Parise G, Vincenti V, Granger HJ, Viglietto G, Persico MG. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Laboratory investigation; a journal of technical methods and pathology. 1997 Apr:76(4):517-31
[PubMed PMID: 9111514]
[27]
Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007 Jan:56(1):224-30
[PubMed PMID: 17192486]
[28]
Lei X, Zhang J, Shen J, Hu LM, Wu Y, Mou L, Xu G, Li W, Xu GT. EPO attenuates inflammatory cytokines by Muller cells in diabetic retinopathy. Frontiers in bioscience (Elite edition). 2011 Jan 1:3(1):201-11
[PubMed PMID: 21196299]
[29]
Garner A. Histopathology of diabetic retinopathy in man. Eye (London, England). 1993:7 ( Pt 2)():250-3
[PubMed PMID: 7607344]
[30]
Crăiţoiu S. [The morphopathological aspects of diabetic retinopathy]. Oftalmologia (Bucharest, Romania : 1990). 1992 Apr-Jun:36(2):141-8
[PubMed PMID: 1525142]
[31]
Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, Wolburg H, Reichenbach A, Bringmann A. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Molecular and cellular neurosciences. 2004 Aug:26(4):493-502
[PubMed PMID: 15276152]
[32]
Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2007 May:245(5):627-36
[PubMed PMID: 17219109]
[33]
Kanski JJ. Diabetic retinopathy--a preventable cause of blindness. The Practitioner. 1985 Apr:229(1402):343-8
[PubMed PMID: 3991450]
[34]
Ezra E, Keinan E, Mandel Y, Boulton ME, Nahmias Y. Non-dimensional analysis of retinal microaneurysms: critical threshold for treatment. Integrative biology : quantitative biosciences from nano to macro. 2013 Mar:5(3):474-80. doi: 10.1039/c3ib20259c. Epub
[PubMed PMID: 23371018]
[36]
. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May:98(5 Suppl):786-806
[PubMed PMID: 2062513]
[37]
. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May:98(5 Suppl):807-22
[PubMed PMID: 2062514]
[38]
Tripathy K, Sharma YR, R K, Chawla R, Gogia V, Singh SK, Venkatesh P, Vohra R. Recent advances in management of diabetic macular edema. Current diabetes reviews. 2015:11(2):79-97
[PubMed PMID: 25801496]
Level 3 (low-level) evidence
[39]
Jorge EC, Jorge EN, Botelho M, Farat JG, Virgili G, El Dib R. Monotherapy laser photocoagulation for diabetic macular oedema. The Cochrane database of systematic reviews. 2018 Oct 15:10(10):CD010859. doi: 10.1002/14651858.CD010859.pub2. Epub 2018 Oct 15
[PubMed PMID: 30320466]
Level 1 (high-level) evidence
[40]
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003 Sep:110(9):1677-82
[PubMed PMID: 13129861]
[41]
Zhang Q, Zhao G, Yang N, Zhang L. Fasting blood glucose levels in patients with different types of diseases. Progress in molecular biology and translational science. 2019:162():277-292. doi: 10.1016/bs.pmbts.2019.01.004. Epub 2019 Mar 6
[PubMed PMID: 30905457]
[42]
Kwan CC, Fawzi AA. Imaging and Biomarkers in Diabetic Macular Edema and Diabetic Retinopathy. Current diabetes reports. 2019 Aug 31:19(10):95. doi: 10.1007/s11892-019-1226-2. Epub 2019 Aug 31
[PubMed PMID: 31473838]
[43]
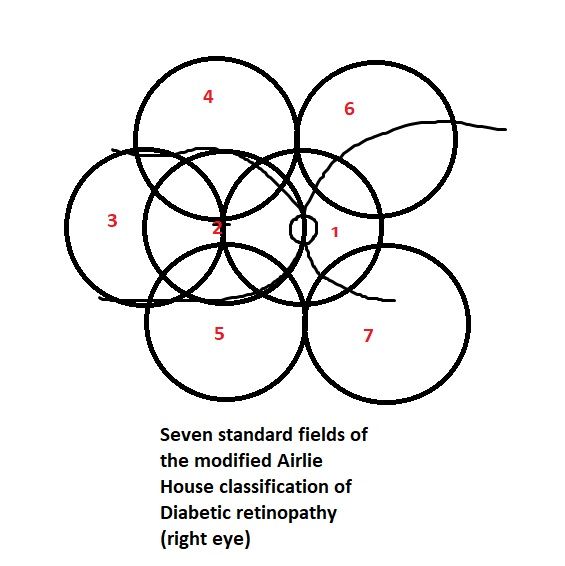
Tripathy K, Chawla R, Wadekar BR, Venkatesh P, Sharma YR. Evaluation of rhegmatogenous retinal detachments using Optos ultrawide field fundus fluorescein angiography and comparison with ETDRS 7 field overlay. Journal of current ophthalmology. 2018 Sep:30(3):263-267. doi: 10.1016/j.joco.2018.06.006. Epub 2018 Jul 3
[PubMed PMID: 30197958]
[44]
Tripathy K, Chawla R, Venkatesh P, Sharma YR, Vohra R. Ultrawide Field Imaging in Uveitic Non-dilating Pupils. Journal of ophthalmic & vision research. 2017 Apr-Jun:12(2):232-233. doi: 10.4103/2008-322X.205360. Epub
[PubMed PMID: 28540019]
[45]
Tripathy K, Chawla R, Vohra R. Evaluation of the fundus in poorly dilating diabetic pupils using ultrawide field imaging. Clinical & experimental optometry. 2017 Nov:100(6):735-736. doi: 10.1111/cxo.12484. Epub 2016 Oct 5
[PubMed PMID: 27704602]
[46]
Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, Turner GS, Stanga PE. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta ophthalmologica. 2013 May:91(3):251-8. doi: 10.1111/j.1755-3768.2011.02307.x. Epub 2011 Dec 16
[PubMed PMID: 22176513]
Level 2 (mid-level) evidence
[47]
Nikkhah H, Ghazi H, Razzaghi MR, Karimi S, Ramezani A, Soheilian M. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. International ophthalmology. 2018 Feb:38(1):313-321. doi: 10.1007/s10792-017-0469-7. Epub 2017 Feb 6
[PubMed PMID: 28168567]
Level 1 (high-level) evidence
[48]
Brown DM, Ou WC, Wong TP, Kim RY, Croft DE, Wykoff CC, DAVE Study Group. Targeted Retinal Photocoagulation for Diabetic Macular Edema with Peripheral Retinal Nonperfusion: Three-Year Randomized DAVE Trial. Ophthalmology. 2018 May:125(5):683-690. doi: 10.1016/j.ophtha.2017.11.026. Epub 2018 Jan 11
[PubMed PMID: 29336896]
Level 1 (high-level) evidence
[49]
Lajmi H, Hmaied W, Othmen AB, Chelly Z, El Fekih L. Optical coherence tomography angiography microvascular changes in diabetics without diabetic retinopathy. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2020 Jul-Sep:34(3):156-159. doi: 10.4103/1319-4534.310404. Epub 2021 Feb 27
[PubMed PMID: 34085005]
[50]
. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May:98(5 Suppl):766-85
[PubMed PMID: 2062512]
[51]
Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Current opinion in ophthalmology. 2017 Nov:28(6):636-643. doi: 10.1097/ICU.0000000000000424. Epub
[PubMed PMID: 28837425]
Level 3 (low-level) evidence
[52]
Vaziri K,Schwartz SG,Relhan N,Kishor KS,Flynn HW Jr, New Therapeutic Approaches in Diabetic Retinopathy. The review of diabetic studies : RDS. 2015 Spring-Summer
[PubMed PMID: 26676668]
[53]
Lattanzio R, Cicinelli MV, Bandello F. Intravitreal Steroids in Diabetic Macular Edema. Developments in ophthalmology. 2017:60():78-90. doi: 10.1159/000459691. Epub 2017 Apr 20
[PubMed PMID: 28427068]
[54]
Kumar A, Tripathy K, Chawla R. Intraocular use of bevacizumab in India: An issue resolved? The National medical journal of India. 2017 Nov-Dec:30(6):345-347. doi: 10.4103/0970-258X.239079. Epub
[PubMed PMID: 30117450]
[55]
Stefanini FR, Arevalo JF, Maia M. Bevacizumab for the management of diabetic macular edema. World journal of diabetes. 2013 Apr 15:4(2):19-26. doi: 10.4239/wjd.v4.i2.19. Epub
[PubMed PMID: 23593532]
[56]
Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007 Oct:114(10):1860-7
[PubMed PMID: 17698196]
Level 1 (high-level) evidence
[57]
Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010 Jun:117(6):1078-1086.e2. doi: 10.1016/j.ophtha.2010.03.045. Epub 2010 Apr 22
[PubMed PMID: 20416952]
Level 1 (high-level) evidence
[58]
Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Archives of ophthalmology (Chicago, Ill. : 1960). 2012 Aug:130(8):972-9
[PubMed PMID: 22491395]
Level 1 (high-level) evidence
[59]
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA, READ-2 Study Group. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009 Nov:116(11):2175-81.e1. doi: 10.1016/j.ophtha.2009.04.023. Epub 2009 Aug 22
[PubMed PMID: 19700194]
[60]
Brown DM, Nguyen QD, Ehrlich JS, RISE and RIDE Research Group. Ranibizumab for diabetic macular edema. Author reply. Ophthalmology. 2013 Jan:120(1):221-2. doi: 10.1016/j.ophtha.2012.07.070. Epub
[PubMed PMID: 23283201]
[61]
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ, RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013 Oct:120(10):2013-22. doi: 10.1016/j.ophtha.2013.02.034. Epub 2013 May 22
[PubMed PMID: 23706949]
[62]
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes care. 2010 Nov:33(11):2399-405. doi: 10.2337/dc10-0493. Epub
[PubMed PMID: 20980427]
Level 1 (high-level) evidence
[63]
Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010 Jun:117(6):1064-1077.e35. doi: 10.1016/j.ophtha.2010.02.031. Epub 2010 Apr 28
[PubMed PMID: 20427088]
Level 1 (high-level) evidence
[64]
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011 Apr:118(4):615-25. doi: 10.1016/j.ophtha.2011.01.031. Epub
[PubMed PMID: 21459215]
[65]
Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Rückert R, Sandbrink R, Stein D, Yang K, Beckmann K, Heier JS. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011 Sep:118(9):1819-26. doi: 10.1016/j.ophtha.2011.02.018. Epub 2011 May 5
[PubMed PMID: 21546089]
[66]
Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014 Nov:121(11):2247-54. doi: 10.1016/j.ophtha.2014.05.006. Epub 2014 Jul 8
[PubMed PMID: 25012934]
[67]
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England journal of medicine. 2015 Mar 26:372(13):1193-203. doi: 10.1056/NEJMoa1414264. Epub 2015 Feb 18
[PubMed PMID: 25692915]
[68]
Cunningham ET Jr,Adamis AP,Altaweel M,Aiello LP,Bressler NM,D'Amico DJ,Goldbaum M,Guyer DR,Katz B,Patel M,Schwartz SD, A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005 Oct
[PubMed PMID: 16154196]
Level 1 (high-level) evidence
[69]
Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS, Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011 Jun:118(6):1107-18. doi: 10.1016/j.ophtha.2011.02.045. Epub 2011 May 6
[PubMed PMID: 21529957]
Level 1 (high-level) evidence
[70]
Brown DM, Emanuelli A, Bandello F, Barranco JJE, Figueira J, Souied E, Wolf S, Gupta V, Ngah NF, Liew G, Tuli R, Tadayoni R, Dhoot D, Wang L, Bouillaud E, Wang Y, Kovacic L, Guerard N, Garweg JG. KESTREL and KITE: 52-Week Results From Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema. American journal of ophthalmology. 2022 Jun:238():157-172. doi: 10.1016/j.ajo.2022.01.004. Epub 2022 Jan 14
[PubMed PMID: 35038415]
[71]
Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, Jampol LM, Melia M, Punjabi OS, Salehi-Had H, Sun JK, Diabetic Retinopathy Clinical Research Network. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA ophthalmology. 2018 Jan 1:136(1):29-38. doi: 10.1001/jamaophthalmol.2017.4914. Epub
[PubMed PMID: 29127949]
Level 1 (high-level) evidence
[72]
Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert opinion on biological therapy. 2016:16(3):365-74. doi: 10.1517/14712598.2016.1131265. Epub 2016 Jan 12
[PubMed PMID: 26674182]
Level 3 (low-level) evidence
[73]
Shah SU, Maturi RK. Therapeutic Options in Refractory Diabetic Macular Oedema. Drugs. 2017 Apr:77(5):481-492. doi: 10.1007/s40265-017-0704-6. Epub
[PubMed PMID: 28197794]
[74]
Jiang AC, Srivastava SK, Hu M, Figueiredo N, Babiuch A, Boss JD, Reese JL, Ehlers JP. Quantitative Ultra-Widefield Angiographic Features and Associations with Diabetic Macular Edema. Ophthalmology. Retina. 2020 Jan:4(1):49-56. doi: 10.1016/j.oret.2019.08.008. Epub 2019 Aug 28
[PubMed PMID: 31690541]
[75]
Khan Z, Kuriakose RK, Khan M, Chin EK, Almeida DR. Efficacy of the Intravitreal Sustained-Release Dexamethasone Implant for Diabetic Macular Edema Refractory to Anti-Vascular Endothelial Growth Factor Therapy: Meta-Analysis and Clinical Implications. Ophthalmic surgery, lasers & imaging retina. 2017 Feb 1:48(2):160-166. doi: 10.3928/23258160-20170130-10. Epub
[PubMed PMID: 28195619]
Level 1 (high-level) evidence
[77]
Usman M. An Overview of Our Current Understanding of Diabetic Macular Ischemia (DMI). Cureus. 2018 Jul 30:10(7):e3064. doi: 10.7759/cureus.3064. Epub 2018 Jul 30
[PubMed PMID: 30280060]
Level 3 (low-level) evidence
[78]
Shah KB, Han DP. Proliferative diabetic retinopathy. International ophthalmology clinics. 2004 Fall:44(4):69-84
[PubMed PMID: 15577565]
[79]
Kansora MB, Goldhardt R. Decision Making in Proliferative Diabetic Retinopathy Treatment. Current ophthalmology reports. 2019 Mar:7(1):45-50. doi: 10.1007/s40135-019-00198-w. Epub 2019 Feb 4
[PubMed PMID: 31595210]
[80]
Sun JK, Glassman AR, Beaulieu WT, Stockdale CR, Bressler NM, Flaxel C, Gross JG, Shami M, Jampol LM, Diabetic Retinopathy Clinical Research Network. Rationale and Application of the Protocol S Anti-Vascular Endothelial Growth Factor Algorithm for Proliferative Diabetic Retinopathy. Ophthalmology. 2019 Jan:126(1):87-95. doi: 10.1016/j.ophtha.2018.08.001. Epub 2018 Aug 7
[PubMed PMID: 30096354]
[81]
El Annan J, Carvounis PE. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. International ophthalmology clinics. 2014 Spring:54(2):141-53. doi: 10.1097/IIO.0000000000000027. Epub
[PubMed PMID: 24613890]
[82]
Wang ZY, Zhao KK, Li JK, Rossmiller B, Zhao PQ. Four-port bimanual 23-gauge vitrectomy for diabetic tractional retinal detachment. Acta ophthalmologica. 2016 Jun:94(4):365-72. doi: 10.1111/aos.12951. Epub 2016 Feb 8
[PubMed PMID: 26855122]
[84]
Dysli M, Rückert R, Munk MR. Differentiation of Underlying Pathologies of Macular Edema Using Spectral Domain Optical Coherence Tomography (SD-OCT). Ocular immunology and inflammation. 2019:27(3):474-483. doi: 10.1080/09273948.2019.1603313. Epub
[PubMed PMID: 31184556]
[88]
Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arquivos brasileiros de oftalmologia. 2013 Oct:76(5):320-7
[PubMed PMID: 24232951]
[89]
Hassan A, Lanzino G, Wijdicks EF, Rabinstein AA, Flemming KD. Terson's syndrome. Neurocritical care. 2011 Dec:15(3):554-8. doi: 10.1007/s12028-011-9555-2. Epub
[PubMed PMID: 21604080]
[90]
Tripathy K. Dissociated optic nerve fiber layer in a case of Terson syndrome. European journal of ophthalmology. 2020 Sep:30(5):NP11-NP14. doi: 10.1177/1120672119853465. Epub 2019 Jun 3
[PubMed PMID: 31155955]
Level 3 (low-level) evidence
[91]
Terelak-Borys B,Skonieczna K,Grabska-Liberek I, Ocular ischemic syndrome - a systematic review. Medical science monitor : international medical journal of experimental and clinical research. 2012 Aug;
[PubMed PMID: 22847215]
Level 1 (high-level) evidence
[92]
Tripathy K, Mazumdar S. Recurrent retinal and choroidal ischemia in a case of ocular ischemic syndrome. Therapeutic advances in ophthalmology. 2019 Jan-Dec:11():2515841419848926. doi: 10.1177/2515841419848926. Epub 2019 Jul 12
[PubMed PMID: 31321381]
Level 3 (low-level) evidence
[94]
Scott IU, Figueroa MJ, Oden NL, Ip MS, Blodi BA, VanVeldhuisen PC, SCORE2 Investigator Group. SCORE2 Report 5: Vision-Related Function in Patients With Macular Edema Secondary to Central Retinal or Hemiretinal Vein Occlusion. American journal of ophthalmology. 2017 Dec:184():147-156. doi: 10.1016/j.ajo.2017.10.008. Epub 2017 Oct 23
[PubMed PMID: 29074161]
[96]
Elyadari M, Slassi N, Ghanem A, Bekkay R, Zarrouki M, Mouine S, Benchakroun N, Berraho A. [Case report of rare post-traumatic subretinal hemorrhage]. Journal francais d'ophtalmologie. 2017 May:40(5):e153-e156. doi: 10.1016/j.jfo.2017.02.003. Epub 2017 May 6
[PubMed PMID: 28483155]
Level 3 (low-level) evidence
[97]
Clemente-Tomás R,Gargallo-Benedicto A,Schilt-Catafal M,García-Ibor F, Retinal arterial macroaneurysm: A study using optical coherence tomography and retinography. Archivos de la Sociedad Espanola de Oftalmologia. 2020 Feb;
[PubMed PMID: 31879142]
[98]
Heydarian S, Jafari R, Dailami KN, Hashemi H, Jafarzadehpour E, Heirani M, Yekta A, Mahjoob M, Khabazkhoob M. Ocular abnormalities in beta thalassemia patients: prevalence, impact, and management strategies. International ophthalmology. 2020 Feb:40(2):511-527. doi: 10.1007/s10792-019-01189-3. Epub 2019 Oct 10
[PubMed PMID: 31602527]
[99]
Schönfeld CL, Schneider T, Körner U, Heidenkummer HP, Kampik A. Prognostic factors in vitreous surgery for proliferative diabetic retinopathy. German journal of ophthalmology. 1994 May:3(3):137-43
[PubMed PMID: 8038681]
[100]
Fernández-Vigo J, Fandiño J, Cordido M. [Prognostic factors in the treatment of edematous diabetic retinopathy with focal photocoagulation]. Ophtalmologie : organe de la Societe francaise d'ophtalmologie. 1988 Oct:2(4):311-4
[PubMed PMID: 3247197]
[101]
Acón D, Wu L. Multimodal Imaging in Diabetic Macular Edema. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 Jan-Feb:7(1):22-27. doi: 10.22608/APO.2017504. Epub 2017 Jan 29
[PubMed PMID: 29376234]
[103]
Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian journal of ophthalmology. 2018 Dec:66(12):1751-1762. doi: 10.4103/ijo.IJO_1217_18. Epub
[PubMed PMID: 30451175]
[104]
Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW, Diabetic Retinopathy Clinical Research Network. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA ophthalmology. 2018 Oct 1:136(10):1138-1148. doi: 10.1001/jamaophthalmol.2018.3255. Epub
[PubMed PMID: 30043039]
Level 1 (high-level) evidence
[105]
Tseng VL, Greenberg PB, Scott IU, Anderson KL. Compliance with the American Academy of Ophthalmology Preferred Practice Pattern for Diabetic Retinopathy in a resident ophthalmology clinic. Retina (Philadelphia, Pa.). 2010 May:30(5):787-94. doi: 10.1097/IAE.0b013e3181cd47a2. Epub
[PubMed PMID: 20168268]
[106]
. Screening guidelines for diabetic retinopathy. American College of Physicians, American Diabetes Association, and American Academy of Ophthalmology. Annals of internal medicine. 1992 Apr 15:116(8):683-5
[PubMed PMID: 1546870]