Continuing Education Activity
Poroma (poroid tumor), a benign adnexal tumor, usually originates from the terminal duct of sweat glands. Initially, the tumor was considered of eccrine origin. However, further research has revealed cases of apocrine, sebaceous, and follicular differentiation. Some authors describe this neoplasm as "acrospiroma," whereas others consider poroma as a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenoma.
Poroma can exhibit potential degenerative progression. Porocarcinoma, its malignant counterpart, typically develops after several years from a preexisting poroma. The exact etiology of poroma is unknown. Unlike other neoplasms of the adnexal follicular lineage, familial propensity has been unidentified as a risk factor for developing poromas. Long-term exposure to radiation is suggested as a potential trigger for the development of both poromas and porocarcinomas. This activity comprehensively describes the epidemiology, genetics, clinical presentation, and management of poroma, providing healthcare professionals with the knowledge and tools to improve patient care.
Objectives:
Identify the clinical and histological features of poromas to facilitate early recognition and diagnosis.
Screen patients with suspected poromas for potential risk factors, such as long-term radiation exposure, and monitor for possible signs of degenerative progression or malignant transformation.
Select optimal management strategies for poromas, including surgical excision or electrosurgical destruction.
Coordinate interdisciplinary care among healthcare team members to ensure accurate diagnosis, facilitate timely intervention, and improve patient outcomes.
Introduction
Poroma (poroid tumor), a benign adnexal tumor, usually originates from the terminal duct of sweat glands.[1] Initially, Pinkus et al described poroma and its differentiation in 1956, which was initially believed to be of eccrine origin.[2] However, further research has revealed cases of apocrine, sebaceous, and follicular differentiation.[3]
Some authors describe this neoplasm as "acrospiroma," whereas others consider poroma as a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenoma.[1] Poroma can exhibit potential degenerative progression. Porocarcinoma, its malignant counterpart, typically develops after several years from a preexisting poroma.[4]
Etiology
The exact etiology of poroma is unknown. Unlike other neoplasms of the adnexal follicular lineage, family predisposition has been unidentified as a risk factor for developing poromas. Long-term exposure to radiation is suggested as a potential trigger for the development of both poromas and porocarcinomas.[5]
Eccrine poromas have also been recently associated with chronic radiation dermatitis.[6] Porocarcinoma can arise as a potential malignant progression of a benign poroma. The pathogenesis remains unclear, and a definitive timeline for the advancement of poroma to porocarcinoma is not evident.[4] Immunosuppression, exposure to chemical agents, and chronic light exposure can increase susceptibility to developing eccrine porocarcinoma.[7]
Poroma is occasionally reported in conjunction with other conditions, such as Bowen disease and hypohidrotic ectodermal dysplasia.[8][9] Porocarcinomas have been reported to occur more frequently in association with xeroderma pigmentosum, extra-mammary Paget disease, Hodgkin lymphoma, chronic lymphocytic leukemia, pernicious anemia, sarcoidosis, and HIV infection.[10][11][12]
Epidemiology
In primary skin lesions, sweat gland tumors account for approximately 1% of cases, whereas eccrine and apocrine poromas are believed to account for approximately 10%.[1][13] Poromas are not associated with any particular ethnicity or race. No sex skew was reported in the distribution. The condition affects males and females equally and can develop at any age, although onset is typically noted in adulthood.[14]
The incidence of eccrine porocarcinoma is lower than that of poroma, representing only 0.005% of epithelial cutaneous neoplasms, and it is more common in older patients.[15] In a meta-analysis published in 2017, eccrine porocarcinoma was diagnosed in 453 patients. In this study, 49% of cases were male, and 51% were female, ranging from 6 months to 97 years.[16]
Pathophysiology
Poroma is a benign adnexal tumor that develops on the skin and does not affect other internal tissues. The cancer exhibits differentiation into glandular ductal cells, also known as poroid cells. Porocarcinoma, its malignant counterpart, demonstrates clear poroid differentiation.[17]
Histopathology
Poroma typically presents as a well-circumscribed tumor.[18] Depending on the location of the tumor cells, poroid tumors are divided into 4 subtypes—eccrine poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor.[13][19] These tumors can be entirely intraepidermal, and this pattern of tumor growth is known as hidroacanthoma simplex. In contrast, dermal duct tumors are poroid tumors predominantly located within or nearly intradermal. Furthermore, eccrine poroma can occur in continuity with the epidermis, called "juxta-epidermal poroma." Poroid hidradenomas are entirely situated within the dermal layer.[1]
Poroid cells are characterized by cuboidal keratinocytes, exhibiting a non-palisading pattern and monomorphic ovoid nuclei with discrete nucleoli. Typically, the cytoplasm appears eosinophilic and stains positively with periodic acid–Schiff (PAS). Interestingly, some atypical features observed in malignant tumors can also be present in poroma, including a variable number of mitoses, highly vascularized stroma, and foci of necrosis. A specific type of poroid tumor is associated with the presence of dendritic melanocytes alongside scattered poroid cells within intraepidermal tumor nests. This histological feature clinically corresponds to a pigmented variant of poroma known as "pigmented hidroacanthoma simplex." [20]
The degree of ductal differentiation varies between poromas. Dermatopathologists can be guided by carcinoembryonic antigen immunostaining—a tool to confirm the presence of ductal differentiation. The staining labels the luminal surface of both apocrine and eccrine ducts.[1]
In their literature review, Kamiya et al proposed 4 histopathological features to distinguish apocrine from eccrine poroma, as mentioned below.[21]
- Elongated tubules lined by columnar cuticular or polygonal cells, often displaying hints of apocrine secretion along the luminal border, contain an amorphous, eosinophilic material within the lumina.
- Aggregates of neoplastic cells connecting to preexisting infundibula, resembling connections between the excretory ducts of apocrine glands.
- Follicular differentiation manifested as epithelial lobules, akin to tumors of the follicular infundibulum or trichoblastoma.
- The presence of isolated sebocytes and small clusters in neoplastic cells.
Porocarcinoma comprises anaplastic cells characterized by large irregular and hyperchromatic nuclei, along with glycogen-rich cytoplasm.[1] In addition, porocarcinoma also exhibits areas of necrosis and the presence of mitotic figures.[22] Usually, porocarcinoma initially proliferates intraepidermally before extending into the dermis. Invasion of dermal lymphatics occurs, and the tumor often demonstrates regional and distant metastasis.
History and Physical
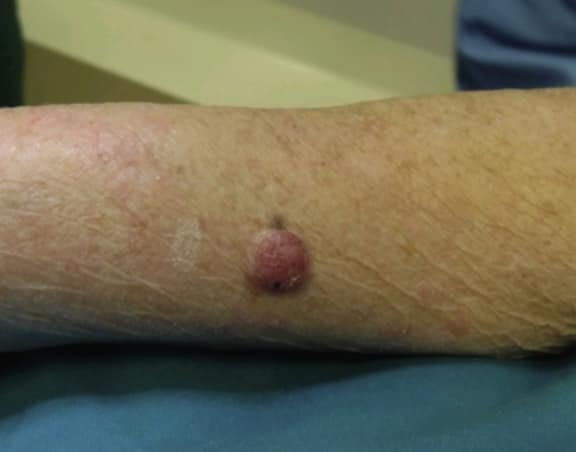
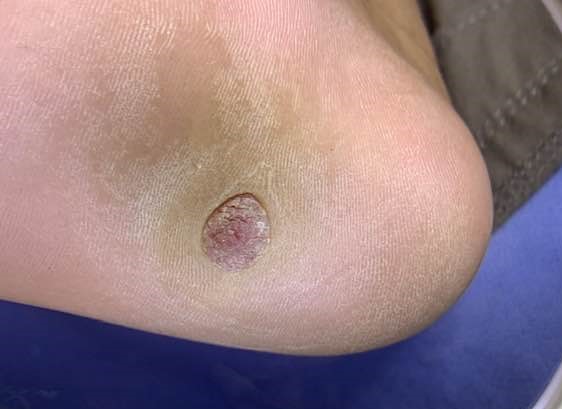
Poroma typically presents as an asymptomatic mild, solitary, slow-growing papule, nodule, or plaque with colors varying from skin color to red to brown or blue (see Image. Nodular Lesion of Poroma).[20] Occasionally, a poroma may exhibit mild tenderness. The lesion commonly displays a vascularized feature, resembling the clinical appearance of a pyogenic granuloma. Indeed, Betti et al identified the reddish color as the most prevalent in a series of 101 cases.[13] Although poromas most frequently occur on the palms and soles of an individual, these lesions can also be found on any cutaneous portion of the entire body surface (see Image. Poroma on a Patient's Sole). Unusual locations include the head, neck, trunk, armpits, upper limbs, buttocks, and lower limbs.
Although sporadic, poroma arising within the nevus sebaceous has also been reported.[23] Apocrine poroma clinically presents similarly to its eccrine correspondent. Nevertheless, none of the reported cases of apocrine poroma was located on palms or soles; they only involved the face, the body, and the limbs.[24]
Multiple poromas are known as "poromatosis," which can occur following chemotherapy and/or radiotherapy.[25] They can be either in an acral or in a widespread distribution. Porocarcinoma may arise due to transformation or as a new lesion (de novo). This is suspected if there is spontaneous recurrent ulceration, bleeding, explosive growth, or sudden pain that occurs in a preexisting tumor.[1] According to a meta-analysis, the most common affected sites are the head and neck (39.9%), followed by the lower extremities (33.9%).[16]
Evaluation
While clinical examination is integral to diagnosis, dermoscopy can further aid in diagnosing poroma. Dermoscopic features associated with poroma include branched vessels with rounded endings, white interlacing areas around vessels, yellow structureless areas, and milky-red globules.[26][27][28] This technique also assists in differentiating between poroma and porocarcinoma. In invasive eccrine porocarcinoma, dermoscopy reveals focal whitish-pink, structureless areas surrounded by pinkish-white halos. Conversely, in eccrine poroma, such areas present a diffuse distribution resembling a "frog-eggs" pattern.[29]
A biopsy is the most definite method for diagnosing poroma. When considering a diagnosis of poroma, attention must be paid to the risk for malignancy. In cases of porocarcinoma, evaluation of the sentinel lymph node biopsy serves as a staging tool, along with screening for distant metastases. Given the variability in clinical and histopathological findings, diagnosing porocarcinoma can be challenging, particularly when histology resembles that of cutaneous squamous cell carcinoma or poroma. Immunohistochemical staining with carcinoembryonic antigen and epithelial membrane antigen may aid in distinguishing porocarcinoma.[30]
Treatment / Management
Treatment for poroma is typically optional but curative, given its benign nature as an adnexal neoplasm. Deeper lesions may be effectively cured with simple excision, while electrosurgical destruction may suffice for superficial lesions. Treatment modalities for porocarcinoma have included Mohs micrographic surgery, standard surgical excision with broad tumor margins, radiation therapy, and chemotherapy.[31] After research, a few authors believe that the Mohs micrographic surgery provides the most excellent possible cure without regional and distant metastases and ensures clear margins.[10]
Differential Diagnosis
Poromas are often misdiagnosed as other skin neoplasms due to their nonspecific and variable clinical presentations. In a study by Chen et al, the preoperative diagnoses of eccrine poroma included pyogenic granuloma (5 cases), soft fibroma (4 cases), verruca vulgaris (3 cases), hemangioma (2 cases), pigmented nevus (1 case), and basal cell carcinoma (1 case). Other potential differentials may include squamous cell carcinoma, seborrheic keratosis, hidradenomas, trichilemmoma, and other adnexal tumors.[1]
Long-standing or chronic tumors affecting the limbs and head, such as squamous cell carcinoma, Paget disease, basal cell carcinoma, and hidradenocarcinoma melanoma, may present with clinical features similar to inflamed poroma and metastatic cancer porocarcinoma.[10] Porocacinoma may resemble an ingrown toenail if it occurs in the nail fold.[4]
Histologically, identifying apocrine poroma can be challenging, as it presents a combination of apocrine, sebaceous, and follicular differentiation within otherwise typical poroid tumors. A typical case of apocrine poroma was reported by Kamiya et al, who discussed its coexistence with basal-cell epithelioma and eccrine poroma in association with trichoblastoma. The study also emphasized the presence of sweat gland differentiation in basal-cell epithelioma.[21]
Prognosis
The prognosis of poromas is generally favorable. Clinically, poromas present as solitary nodules or papules. These lesions are typically asymptomatic, although some may exhibit mild tenderness. In some cases, patients may develop multiple poromas simultaneously—a condition known as "porokeratosis." Poromatosis can be a cosmetic concern for the patient.
The risk of malignant transformation from poroma to porocarcinoma is minimal. In a study by Salih et al., metastasis of porocarcinoma was detected at presentation in 31% of cases. The most common sites for metastasis of porocarcinoma in the body were neighboring lymph nodes (57.7%), followed by the respiratory tract or lungs (12.8%), brain (9%), liver (9%), skin (5.8%), bone (3.2%), stomach (0.6%) and breast (0.6%). Disseminated metastasis was observed in 1.3% of cases.[16][32][16]
Complications
As a benign condition, poroma is not inherently associated with specific complications. However, common complications, such as infection and hemorrhage, may arise following surgical procedures.
Consultations
Poromas are benign tumors that lack distinct clinical features and can be challenging to diagnose. However, experienced clinical dermatologists can often identify poromas, particularly those presenting with a vascular appearance on a volar surface.
Deterrence and Patient Education
Poroma is typically a benign tumor, often asymptomatic, with a generally favorable prognosis. Patient education regarding malignant transformation, such as porocarcinoma, is usually not necessary because the risk is comparable to that of normal skin. However, poromatosis can lead to cosmetic disfigurement and, when affecting the soles, may result in some degree of disability.
Enhancing Healthcare Team Outcomes
Poroma is a benign tumor typically associated with mild clinical symptoms, although rare progression to porocarcinoma can occur. An interprofessional healthcare team comprising clinicians, dermatologists, histopathologists, and surgical specialists is crucial to diagnosing and managing poromas in patients. Close collaboration and communication among healthcare professionals help ensure coordinated care and shared decision-making in addressing the clinical symptoms associated with this condition.
Skin biopsy for histopathological analysis is indicated in all cases with suspected poroma to rule out its malignant variant. Although porocarcinoma is a rare malignancy, it is curable with accurate diagnosis and appropriate treatment.