Continuing Education Activity
Pernicious anemia is a relatively rare autoimmune disorder that causes diminishment in dietary vitamin B12 absorption, resulting in B12 deficiency and subsequent megaloblastic anemia. It affects people of all ages worldwide, particularly those over 60. Despite the advances in understanding, making the diagnosis can be challenging for clinicians due to its complexity, broad spectrum of clinical presentation, and limitations of the currently available diagnostic tests. Once diagnosed, prompt treatment with B12 supplementation commonly reverses the patient's anemia; however, they will require lifelong supplementation and monitoring. This activity reviews the etiology, evaluation, and treatment of pernicious anemia and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
Objectives:
- Describe the etiology of pernicious anemia.
- Select diagnostic testing based on clinical evidence.
- Implement the guideline-recommended treatment for patients with pernicious anemia.
- Explain the roles of an interprofessional team in the evaluation and management of patients with suspected pernicious anemia.
Introduction
Pernicious anemia is a relatively rare autoimmune disorder that causes diminishment in dietary vitamin B12 (cobalamin) absorption, resulting in B12 deficiency and subsequent megaloblastic anemia. It affects people of all ages worldwide, particularly those over 60. Despite the advances in understanding, making the diagnosis can be challenging for clinicians due to its complexity, broad spectrum of clinical presentation, and limitations of the currently available diagnostic tests. Once diagnosed, prompt treatment with B12 supplementation commonly reverses the patient's anemia; however, they will require lifelong supplementation and monitoring.
Etiology
Pernicious anemia is a complex disease, with a clear autoimmune basis. The anemia is megaloblastic and is caused by vitamin B12 deficiency secondary to intrinsic factor (IF) deficiency.[1] IF is a glycoprotein produced and secreted by parietal cells that binds B12 and facilitates its transport to the terminal ileum for absorption. Anti-IF antibodies inhibit B12 from binding to IF, preventing B12/IF complex formation or binding to the B12/IF complex, preventing intestinal absorption.
Pernicious anemia is found in up to 25% of patients with autoimmune gastritis (AIG), which affects the corpus and fundus, spares the antrum, and is characterized by antiparietal cell antibodies destroying parietal cells located only in the oxyntic mucosa, which produce IF and hydrochloric acid. In turn, achlorhydria causes a decrease in the release of cobalamin bound to dietary protein, and fewer parietal cells are available to produce the IF needed for dietary B12 absorption. The term pernicious anemia has been used at times as a synonym for AIG; however, it is considered a late-stage manifestation as a part of the AIG clinical spectrum.[2][3][4]
Patients with pernicious anemia also have a higher incidence of co-occurring autoimmune disorders, including diabetes mellitus type 1, autoimmune thyroid disease, and vitiligo.[1][5][6][7] Furthermore, 5 genome-wide significant signals for pernicious anemia have been identified, providing evidence for genetic risk factors.[8]
Whether or not Helicobacter pylori plays a causative role in pernicious anemia is unclear.[9]
Epidemiology
Pernicious anemia is a relatively rare condition, with a prevalence of less than 1% in populations of European ancestry. Worldwide, pernicious anemia is a common cause of megaloblastic anemia; it affects people of all ages, particularly those over 60-70 (~2% prevalence); it affects both sexes with a varying geographical female-to-male ratio; and the prevalence is lower in those of Asian descent compared to other studied populations.[10][11][12]
Pathophysiology
The primary pathophysiological mechanism in pernicious anemia is diminished dietary B12 absorption due to IF deficiency. Vitamin B12 is found in meat, eggs, and dairy products and is essential for erythropoiesis and nerve myelination; therefore, its deficiency can lead to megaloblastic anemia due to disrupted DNA synthesis and demyelinated nerves. B12 deficiency causes megaloblastic changes in all formed blood elements, but erythrocytes show the most significant changes, with the degree of anemia corresponding to the severity of red blood cell morphologic changes.[13]
Dietary B12 is released from food carrier proteins via proteolysis in the acidic gastric environment and binds to haptocorrin, protecting B12 from degradation.[14] B12 is released from haptocorrin in the small intestine by pancreatic proteases and binds to IF produced by gastric parietal cells. The IF-B12 complex binds to cubam receptors in the terminal ileum, is endocytosed, binds to transcobalamin, then is released in the bloodstream and delivered to transcobalamin cell receptors. After cellular uptake and intracellular release, B12 is converted to adenosylcobalamin and methylcobalamin, where they serve as cofactors for the two B12-dependent enzymatic reactions.[15]
Vitamin B12 deficiency prevents the proper functioning of these coenzymes, leading to an accumulation of their substrate in plasma. Specifically, adenosylcobalamin deficiency results in an accumulation of methylmalonic acid (MMA), and methylcobalamin deficiency in an accumulation of homocysteine. Additionally, the formation of S-adenosylmethionine (SAM) and methionine diminish.[16][17]
SAM is the methyl donor to key substrates, such as nucleic acids, proteins, phospholipids, and neurotransmitters. As a donor in DNA methylation, it plays an important role in epigenetic adaptability to developmental and environmental factors.[18] Homocysteine excess can cause cellular stress, apoptosis, and homocysteinylation-induced structural and functional alterations in proteins.[19] Moreover, elevated plasma total homocysteine is associated with white matter damage, neurofibrillary tangles, brain atrophy, cognitive decline, and dementia.[20] The effects of accumulated MMA are unclear.[16]
History and Physical
Pernicious anemia develops slowly, with a progression time to apparent clinical B12 deficiency of 2 to 5 years.[21] Symptoms may not manifest until the anemia is relatively profound because compensatory cardiopulmonary mechanisms facilitate increases in oxygen delivery. Due to its insidious nature and the wide array of potential presenting symptoms, a high index of clinical suspicion is often necessary for the judicious use of appropriate testing to make a prompt diagnosis. Patients may present with constitutional, neurological, psychiatric, otolaryngologic, cardiopulmonary, and/or gastrointestinal symptoms, which are often present in other conditions, resulting in a missed or delayed diagnosis.[1][22][23]
- Constitutional: fatigue, lethargy, anorexia, weight loss
- Neurological: headache, confusion, difficulty concentrating, memory loss, cognitive decline, paraesthesias, numbness, imbalance
- Psychiatric: emotional lability, depression, personality changes, psychosis
- Otolaryngologic: hypogeusia/ageusia, glossitis
- Cardiopulmonary: palpitations, dyspnea
- Gastrointestinal: dyspepsia, diarrhea, decreased appetite
The physical examination may be notable for pallor, dry skin, jaundice, glossitis (tender, smooth, red tongue), and tachycardia. In patients of European ancestry, “the melding of severe pallor with jaundice caused by hemolysis produces a peculiar lemon-yellow skin color.”[13]
Peripheral neuropathy is an early neurological manifestation, which may be followed by subacute combined degeneration (SCD) of the spinal cord in later stages. It is typically symmetric and affects the legs more than the arms. Examination shows a decrease in sensitivity to light touch, pinprick, and vibration. Patients with severe loss of position sense may demonstrate a positive Romberg test. Deep tendon ankle reflexes are commonly hypoactive or absent, while more proximal reflexes may still be intact. Importantly, neurologic findings may be present in the absence of anemia. Examination of a patient with SCD will likely demonstrate limb weakness and ataxia, and they may report visual disturbances. The prompt recognition of SCD is important because early diagnosis and treatment improve the chances of recovery.[24]
A neuropsychiatric evaluation may be required.[25] Additionally, the clinician should inquire whether the patient, a parent, or a sibling has diabetes mellitus type 1, vitiligo, or hypothyroidism because a positive personal or family history of an autoimmune condition increases the pretest probability of pernicious anemia.[26]
Evaluation
Making a definitive diagnosis of pernicious anemia can be “problematic” due to the Schilling test becoming obsolete and the absence of a currently approved B12 absorption test.[13] Furthermore, there are multiple diagnostic algorithms and varying diagnostic criteria.[1][22][23][27][28][29] Evaluation will likely include a combination of the following:
- Initial serology: complete blood count (CBC), cobalamin level, folate level, iron panel (serum iron, total iron-binding capacity, ferritin), reticulocyte count
- Peripheral blood smear
- Follow-up serology: anti-IF antibodies, antiparietal cell antibodies
- Additional serology: MMA, fasting homocysteine, holotranscobalamin (HTC)
- Bone marrow biopsy
- Endoscopy with biopsies
A CBC may be significant for anemia, macrocytosis (mean corpuscular volume ≥100 fl), and pancytopenia (due to ineffective erythropoiesis and myelopoiesis). However, about one-third of patients with B12 deficiency may not have macrocytosis.[1][22] Normocytic anemia can be seen if there is concomitant iron deficiency anemia, a complication of achlorhydria, or another condition causing microcytosis that can mask the macrocytosis. Iron deficiency may precede a pernicious anemia diagnosis or be concomitant with it.[1][28] Moreover, pernicious anemia can present with nonanemic macrocytosis months before the diagnosis and neurologic signs and symptoms can occur in the absence of anemia or macrocytosis in 25 to 30% of patients eventually diagnosed with pernicious anemia.[1][29] A reticulocyte count may show a reduction in the absolute number of reticulocytes.
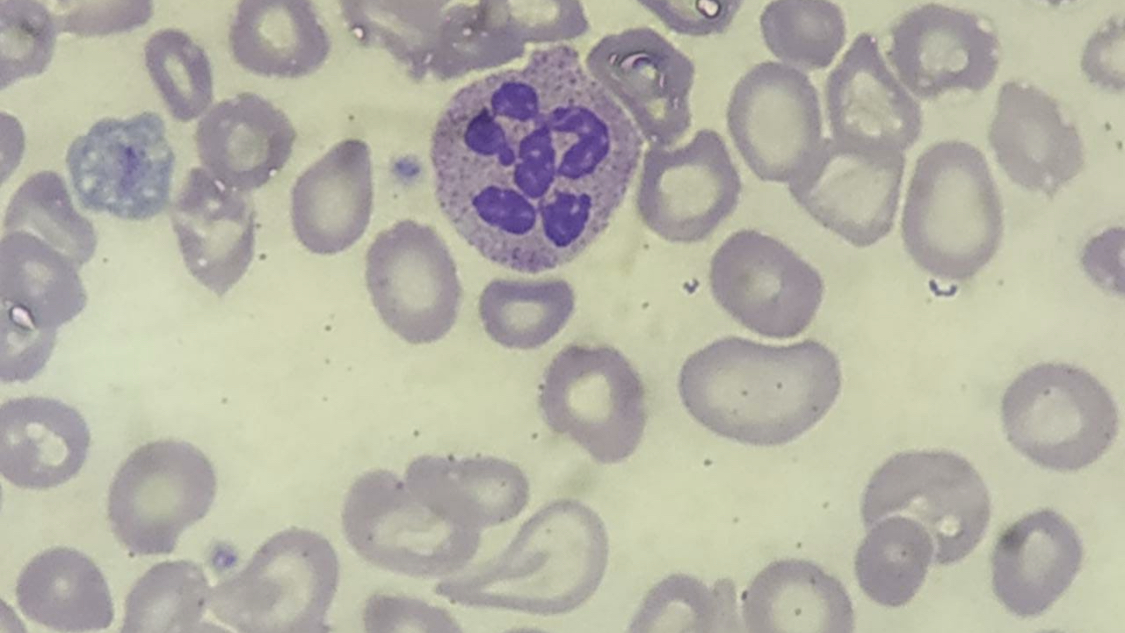
A peripheral blood smear may show macro-ovalocytes, hypersegmented neutrophils, and anisopoikilocytosis. Although hypersegmented neutrophils are often considered a hallmark of megaloblastic anemia, they are not specific, as they can be seen in other types of anemia, like iron deficiency anemia. Hypersegmented neutrophils typically precede macrocytosis and anemia; however, in advanced disease, they may be rare or absent.
An isolated serum cobalamin level has poor sensitivity and specificity for reliably detecting B12 deficiency.[13] B12 deficiency is defined by a serum cobalamin level of <200 ng/L in the appropriate clinical context. Patients with pernicious anemia and serum cobalamin levels ≥200 ng/L may have true B12 deficiency because levels can be falsely elevated in up to 35% of these patients.[1] In 2018, authors reviewing evidence on “methods to assess B12 bioavailability and technologies to enhance its absorption” concluded that “in most cases, the evidence of absorption enhancement is limited.”[30] The CobaSorb test has shown promise, but it does not discriminate between gastric and intestinal causes of B12 malabsorption, and it cannot be used once a patient is treated with B12.[31][32]
In patients with serum cobalamin levels ≥200 ng/L diagnostic performance may be enhanced by measuring serum MMA, fasting homocysteine, and HTC.[1] Elevated MMA and/or fasting homocysteine are indicators of B12 deficiency in patients without evidence of impaired renal function or an inherited cobalamin processing enzyme defect. MMA is more sensitive and more specific, and elevation is seen only in patients with B12 deficiency. Homocysteine levels may also increase in folate deficiency, pyridoxine deficiency, and hypothyroidism. Serum HTC levels may be used for assessing B12 status, with or without MMA and/or homocysteine, and may reflect B12 absorptive capacity.[13]
Folate levels should be determined to exclude macrocytic anemia secondary to folate deficiency and because treating B12-deficient patients with folate alone may worsen associated neurologic damage.[23] Iron deficiency anemia is present in up to 50% of patients with AIG, and pernicious anemia is present in up to 25% of patients with AIG[2]; therefore, an iron panel is indicated in patients diagnosed with pernicious anemia.
Follow-up serology will include anti-IF antibodies with or without antiparietal cell antibodies. Anti-IF antibodies are 40 to 60% sensitive in detecting pernicious anemia, with the rate of positivity rising with disease progression. The specificity of anti-IF antibody testing is almost 100%. Although antiparietal cell antibodies are present in 90% of patients with pernicious anemia, they are less specific than anti-IF antibodies. Notably combining the two has been shown to significantly increase their diagnostic performance for pernicious anemia (73% sensitivity and 100% specificity).[28][33]
A serum cobalamin of <200 ng/L plus the presence of anti-IF antibodies confirms a diagnosis of pernicious anemia.
According to the British Committee for Standards in Hematology, all patients with anemia, neuropathy, or glossitis, and suspected of having pernicious anemia, should be tested for anti-IF antibodies regardless of cobalamin levels. Secondly, the committee recommends anti-IF antibody testing in patients found to have a low serum cobalamin level in the absence of anemia and who do not have food malabsorption or other causes of deficiency to clarify whether they have an early or latent presentation of pernicious anemia. Thirdly, antiparietal cell antibody testing for diagnosing pernicious anemia is not recommended.[26]
Of note, there are two types of IF autoantibodies: type 1 are “blocking autoantibodies” that inhibit B12 from binding to IF, preventing B12/IF complex formation, and type 2 are “binding autoantibodies” that bind to the B12/IF complex, preventing intestinal absorption. Type 1 is used in evaluating suspected pernicious anemia because it is highly specific and is the type present in approximately 70% of patients.[13][29]
Histological examination after a bone marrow biopsy is usually unnecessary. When performed, it will show hypercellularity with a shift toward immaturity, including abnormal maturation of myeloid and erythroid cell lines.[1][23]
As mentioned earlier, pernicious anemia is found in up to 25% of patients with AIG as a late-stage manifestation. Because autoimmune chronic atrophic gastritis is characterized by an elevated fasting serum gastrin level, the fasting serum gastrin assay can play a role in establishing a pernicious anemia diagnosis in difficult cases.[27][28] Moreover, a deficiency in gastric intrinsic factor output after pentagastrin stimulation is specific for the diagnosis of pernicious anemia and can be used when evaluating serological markers in certain patients.[34]
According to a 2021 American Gastroenterological Association clinical practice update, patients with a new pernicious anemia diagnosis who have not had a recent endoscopy should undergo endoscopy with topographical biopsies to confirm corpus-predominant atrophic gastritis for risk stratification and to rule out prevalent gastric neoplasia, including neuroendocrine tumors.[3]
Treatment / Management
Lifelong treatment for patients with confirmed pernicious anemia starts with an intramuscular (IM) injection of 1000 micrograms of B12 (hydroxocobalamin in Europe or cyanocobalamin in the United States) administered daily or every other day for 1 to 2 weeks, followed by weekly injections for 1 to 2 months, then a monthly injection (cyanocobalamin) or every 2 to 3 months (hydroxocobalamin).[11][16][21][26]
Following the initial intensive treatment phase, patients can continue IM injections, or they can be offered high-dose oral B12 supplementation for the lifelong maintenance phase. In a 2016 review of oral B12 replacement for treating pernicious anemia, the authors concluded that "oral vitamin B12 is an effective alternative to vitamin B12 IM injections."[35] High-dose cyanocobalamin (1000–2000 micrograms) is most commonly taken daily as an oral tablet. Alternate formulations include sublingual and intranasal, but they are not routinely recommended.[11][16][21][26]
Differential Diagnosis
Other causes of megaloblastic anemia:
- myelodysplastic syndromes
- hemolytic anemia
- thrombotic thrombocytopenic purpura
Other causes of B12 deficiency:
- food-cobalamin malabsorption
- veganism
- metformin
- long-term proton pump inhibitor therapy
- estrogen contraceptive pills
- gastric surgery
- ileal disease
- ileal resection
Other causes of macrocytosis:
- folate deficiency
- methotrexate
- alcohol use disorder
Other causes of peripheral neuropathy:
- diabetes mellitus
- carpal tunnel syndrome
- infections
- medications
- other vitamin deficiencies
- alcohol use disorder
Prognosis
Before the discovery of treatment, pernicious anemia could be fatal.[13] The prognosis since has been excellent with appropriate management, except for patients diagnosed with SCD. Although B12 supplementation stops progression and improves neurologic deficits in most patients with SCD, evidence shows complete resolution only occurs in a small percentage of them.[24] After treatment initiation for pernicious anemia, reticulocytosis begins approximately 5 days later, followed by red blood cell count normalization within 4 to 6 weeks.[11] Typically, neurological symptom improvement is slower than hematological improvement, and the degree of neurological recovery is inversely proportional to the severity and duration of symptoms before treatment. Psychiatric symptoms such as emotional lability and psychosis may rapidly improve.[36]
Complications
Patients with pernicious anemia are at an increased risk of gastric cancer. An international meta-analysis of over 22,000 patients with pernicious anemia found a pooled gastric cancer incidence of 0.27% per person-year. The same study showed a pooled gastric cancer recurrence rate of 6.8.[37]
A large United States population study based on a Surveillance, Epidemiology, and End Results (SEER) Medicare database found an increased risk of 10 cancer types. The authors state, “The most striking of our findings was an 11-fold increase in the risk of gastric carcinoid tumors for both men and women with pernicious anemia, a risk that was higher among cases occurring 6 or more years after the pernicious anemia report/diagnosis.” They also point out these are uncommon cancers with low absolute risk: “Of the 17,076 cancers in our study among people with pernicious anemia, just 83 (0.5%) were gastric carcinoid tumors.”[38]
Management Guidelines
British Society of Gastroenterology (2019): "We suggest that a baseline endoscopy with biopsies should be considered in individuals aged ≥50 years, with laboratory evidence of pernicious anemia, defined by vitamin B12 deficiency and either positive gastric parietal cell or intrinsic factor antibodies. As gastric adenocarcinoma affects the corpus in pernicious anemia, biopsies should be taken from the greater and lesser curves (evidence level: low quality; grade of recommendation: weak; level of agreement: 93%)."[39]
European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) (2019): "Patients with autoimmune gastritis (includes those with pernicious anemia) may benefit from endoscopic follow-up every 3 to 5 years. Low-quality evidence, weak recommendation (82% agree [76% strongly or moderately agree])."[40]
American Gastroenterological Association (2021): "Providers should recognize pernicious anemia as a late-stage manifestation of autoimmune gastritis that is characterized by vitamin B-12 deficiency and macrocytic anemia. Patients with a new diagnosis of pernicious anemia who have not had a recent endoscopy should undergo endoscopy with topographical biopsies to confirm corpus-predominant atrophic gastritis for risk stratification and to rule out prevalent gastric neoplasia, including neuroendocrine tumors."[3]
Deterrence and Patient Education
Patient education is primarily focused on treatment; however, patients should also be advised about their increased risk of cancer, particularly gastric, and pernicious anemia’s association with other autoimmune disorders. Clinicians must recognize proper adherence to lifelong treatment and monitoring requires consideration of the patient’s preferred route of vitamin B12 supplementation. Patients choosing oral therapy may confuse it with optional self-supplementation, and those who choose IM injections may require education regarding self-administration. Furthermore, patients may inadvertently discontinue treatment when their symptoms improve or resolve. Ongoing patient education improves the chances of patients adhering to the necessary lifelong treatment and monitoring. They must also be educated on the signs and symptoms of other autoimmune disorders and gastric malignancies to improve their chances of earlier diagnosis and treatment.
Enhancing Healthcare Team Outcomes
Patients with pernicious anemia can present in one or more clinical settings due to this insidious condition's wide array of potential manifestations. They may be seen by providers in the area(s) of nutrition, primary care, hematology, gastroenterology, neurology, psychiatry, rheumatology, and endocrinology, to name a few. Making the correct diagnosis will include laboratory scientists and may also include pathologists. Once diagnosed, the patient's care may involve a medical assistant, nurse, physician assistant, nurse practitioner, physician, and pharmacist.
Patients require a lifelong coordinated effort by an interprofessional team to enhance healthcare team outcomes. For example, a hematologist prescribing B12 supplementation and ordering bloodwork, a medical assistant administering IM B12 injections, a gastroenterologist providing surveillance for gastric cancer, an endocrinologist treating diabetes mellitus, and a pharmacist reconciling medications.[Level 5]