Continuing Education Activity
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers. The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. This activity reviews the epidemiology, presentation, and complications of Plasmodium malaria and the role of the interprofessional team in evaluating and managing patients with this life-threatening infection.
Objectives:
Review the epidemiology of malaria infection.
Describe the pathophysiology of malaria infection.
Summarize the pharmacologic treatment strategies for malaria infection.
Outline the importance of collaboration amongst an interprofessional team to improve outcomes for patients receiving malaria treatment.
Introduction
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year.[1] The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. Preferred antimalarial therapeutic and chemoprophylactic regimens get dictated by species, geography, susceptibility, and patient demographics. Latent or reactivating infections may be reported years following exposure.
Etiology
The incubation period, and therefore time to symptom development, varies by species: 8 to 11 days for P. falciparum, 8 to 17 days for P. vivax, 10 to 17 days for P. ovale, 18 to 40 days for P. malariae (though possibly up to several years), and 9 to 12 days for P. knowlesi.[1] The periodicity of the Plasmodium lifecycle creates the classic "malarial paroxysm" of rigors, followed by several hours of fever, followed by diaphoresis, and a drop to normal body temperature (P. vivax infection establishes a 48-hour cycle), though this is less commonly seen today due to rapid identification and treatment.[1]
Epidemiology
Forty percent of the global population resides in or visits malaria-endemic regions annually.[1] P. falciparum is present in Western and sub-Saharan Africa and displays the highest morbidity and mortality of the Plasmodia species.[2] P. vivax is present in South Asia, the Western Pacific, and Central America.[2] P. ovale and P. malariae are present in Sub-Saharan Africa.[2] P. knowlesi is present in Southeast Asia.[2] As many as 500 million malaria cases occur annually, with 1.5 to 2.7 million deaths.[1] Ninety percent of fatalities occur in Africa.[1] Those at highest risk include children under age 5, pregnant women, and disease naïve populations, including refugees in Central and Eastern Africa, nonimmune civilian and military travelers, and immigrants returning to their place of origin.[2]
Of the 125 million travelers who visit endemic locations each year, 10000 to 30000 develop malaria, and 1% of these will die from complications of their disease.[2][3] Rising average global temperatures and changes in weather patterns are projected to expand the burden of malaria; a rise of 3 degrees Celsius is postulated to increase malaria incidence by 50 to 80 million.[1]
Pathophysiology
Five Plasmodium species possess the ability to infect humans: P. falciparum, P. ovale, P. vivax, P. malariae, and P. knowlesi.[2] The female Anopheles mosquito ingests gametes during a blood meal, which form sporozoites that replicate in the gut.[1] During subsequent bloodmeals, saliva containing sporozoites gets released into a human host's bloodstream.[1] Within 60 minutes, sporozoites reach the liver, invade hepatocytes, and then rapidly divide, forming merozoites. In an active infection, organisms reenter the bloodstream and invade erythrocytes.[1][4] Within erythrocytes, Plasmodia consume hemoglobin and develop from immature trophozoites (ring stage) to either mature trophozoites or gametocytes (CDC Malaria 2019). Mature trophozoites replicate, forming schizonts, disrupting erythrocyte cell membrane integrity, and leading to capillary endothelial adherence and cell lysis.[1]
Free heme is released into the peripheral blood, which stimulates endothelial activation.[5][6] Untreated malaria lasts 2 to 24 months.[1] P. vivax and P. ovale infections may display "dormant schizogony," where inactive intrahepatic parasites (hypnozoites) remain until reactivation months to years in the future.[1] Although hypnozoite parasites do not routinely develop in the liver in the setting of P. falciparum and P. malariae infection, there are few reports of resurgent P. falciparum infection years after initial exposure.[7]
Pathogenesis stems from toxin-induced IFN-gamma and TNF-alpha secretion.[8] The innate immune response is dominated by monocyte and macrophage phagocytosis within the splenic red pulp. Adaptive immunity develops by IFN-gamma and TNF-alpha-induced class switching of CD4-positive lymphocytes.[4] TNF also suppresses hematopoiesis, which contributes to anemia. The liver and spleen enlarge, causing massive splenomegaly.[8]
Low arginine, low nitric oxide, and elevated arginase activity have been observed in severe malaria in peripheral blood.[9] Studies have shown that the parasite's arginase enzyme may contribute to low arginine in severely ill patients, thus reducing nitric oxide production. Low nitric may lead to subsequent pulmonary hypertension and myocardial wall stress in children. Therefore, peripheral arginine or inhaled nitric oxide are possible treatment options.[10]
Parasitemia dictates symptom onset and severity: symptoms typically develop with 0.002% parasitemia in naïve patients and 0.2% parasitemia in previously exposed patients.[1] Severe infection usually exhibits parasitemia of 5%.[1][4]
Histopathology
Intracellular digestion of hemoglobin by parasites forms hemozoin and makes the membrane less deformable, which results in hemolysis or splenic clearance.
History and Physical
In taking a history, it is essential to inquire about the location of residence, recent travel and use of chemoprophylaxis, exposures (including sick contacts, fresh water, caves, farm/wild animals, insects/arthropods), HIV status, history of current or recent pregnancy, history of G6PD deficiency, history of sickle cell disease, history of anemia, history of blood or other cancers, and history of prior malarial infections (including successful or failed treatments).
Fever is the dominant symptom of malaria—fever, especially for seven or more days, in a patient residing in or with recent travel to an endemic region is highly suspicious and should prompt evaluation.[3] Adults may exhibit headaches, malaise, weakness, gastrointestinal distress, upper respiratory symptoms, and muscle aches; severe cases may include jaundice, confusion, seizures, and dark urine.[2][1] Children are more likely to present with non-specific or gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence.[2] They are more likely to develop hepatomegaly, splenomegaly, and severe anemia without major organ dysfunction than adults. In the case of severe malaria, they present with more frequent seizures (60 to 80%), hypoglycemia, and concomitant sepsis but are less likely to develop pulmonary edema and renal failure than adults.[11][2]
Pregnant Women
The clinical features of infection in pregnancy vary from asymptomatic to severe, depending on the degree of (incomplete) immunity that a woman had acquired by the time she got pregnant. In semi-immune pregnant women, only a few infections result in fever or other symptoms.[12] Malaria in pregnancy has a devastating effect on maternal health and has been associated with increased infant mortality due to low birth weight caused by either intrauterine growth restriction or preterm labor, or both.[12] P. falciparum infections are associated with complications such as maternal anemia, low birth weight, miscarriage, stillbirths, and congenital malaria.[13][12] It is more likely for a pregnant woman in the second or third trimester to develop severe malaria with complications such as hypoglycemia and pulmonary edema compared to non-pregnant adults.[14]
Evaluation
Initial evaluation of undifferentiated fever in stable patients with possible malaria exposure includes a complete blood count, comprehensive metabolic panel, coagulation panel, blood culture, urinalysis, chest radiograph, and thick and thin blood smears. In patients with altered mental status, when cerebral malaria is suspected, a lactate level, arterial blood gas, and lumbar puncture may also be indicated.[2]
In patients with malaria, complete blood count reveals thrombocytopenia in 60-70% of all cases and varying degrees of anemia in 29% of adults and 78% of children.[2] Anemia is more severe in P. falciparum due to invasion of all aged erythrocytes and capillary and splenic erythrocyte sequestration secondary to decreased flexibility and cytoadherence.[1] Anemia is typically moderate with P. vivax and P. malariae due to preferential invasion of reticulocytes and older erythrocytes, respectively.[1] A comprehensive metabolic panel may reveal hepatocellular injury secondary to parasitic invasion, indirect hyperbilirubinemia due to hemolysis, electrolyte abnormalities secondary to the release of intracellular contents, concomitant dehydration, and kidney injury secondary to glomerular damage.[2] The coagulation panel may reveal coagulopathy concerning bleeding risk in patients with severe thrombocytopenia or liver dysfunction. Urinalysis may show proteinuria indicative of nephrotic syndrome.[1]
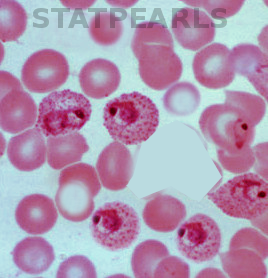
The gold standard for malaria diagnosis is a microscopic evaluation of Giemsa-stained thick and thin smears of a free-flowing venipuncture blood specimen.[2][1] Examination with oil immersion must be completed at 100-times and 1000-times magnification to avoid missing low-level parasitemia or "delicate ring forms."[1] The extent of parasitemia is estimated by the number of organisms per high-powered field.[1] Varying microscopic appearance of infected erythrocytes guides speciation:
- The ring stage in P. falciparum appears as a "purple spot with a thin ring;" in P. vivax as a "purple spot with a deformed body;" in P. ovale as a "ring with a large purple spot;" in P. malariae as a "purple spot with a thick body;" and in P. knowlesi as a "purple spot (or spots) with an amorphous thick ring."[15]
- The trophozoite stage in P. falciparum appears as "a bigger spot [growing] around a smaller spot;" in P. vivax as "a misshapen circle which contains an extended spot;" in P. ovale as "an oval circle (sometimes with small corners) which contains a purple spot with undefined shapes;" in P. malariae as "basket or band-shaped [without a] spot;" and in P. knowlesi as a "purple branched spot."[15]
- The schizont stage in P. falciparum is not established; in P. vivax, it appears as "not defined purple spots inside a circle;" in P. ovale as "more than one spot inside an oval circle (sometimes with small corners);" in P. malariae as "diffuse purple spots around a darker spot;" and in P. knowlesi as "defined purple spots [that are] easy to count."[15]
- The gametocyte stage in P. falciparum appears as "banana [or] sausage-shaped;" in P. vivax as an "extended, big spot;" in P. ovale as a "row of accumulated spots;" in P. malariae as a "big stained spot which almost fills[s] the circle;" and in P. knowlesi as a "big spot which contains small spots."[15]
An initial negative smear does not rule out malaria, as infected erythrocytes may become intravascularly sequestered; if clinical suspicion of malaria is high, smears require repetition in 12 and 24 hours.[2] The malarial pigment in monocytes and neutrophils may also manifest on the blood smear, particularly in patients with cerebral malaria.[1]
Other diagnostic modalities include rapid diagnostic testing (RDT), microhematocrit centrifugation, and polymerase chain reaction (PCR). RDTs detecting parasitic antigens histidine-rich-protein-2, lactate dehydrogenase, and aldolase are increasingly being utilized to diagnose P. falciparum infection.[2][16] Sensitivities approach 100%, though microscopy is still a recommendation at the time of presentation and 12 and 24 hours. Limitations of RDTs include the detection of P. falciparum species only, the inability to quantify parasitic burden, and false-positive results occurring weeks after infection due to persistent blood antigens.[2] Microhematocrit centrifugation isolates infected erythrocytes, then binds to acridine in the collection tube, causing the fluorescence of parasites.[1] PCR is useful in low-level parasitemia detection and speciation.
Treatment / Management
Treatment for patients diagnosed with malaria includes schizonticidal medications, supportive care, and hospitalization for high-risk patients. Naïve adult and pediatric patients receiving active antimalarial treatment should remain inpatient for at least 24 hours to ensure adequate and correctly timed medication dosing and to trend parasitemia to evaluate treatment response. Higher initial parasitemia and poor downtrend are associated with fluid imbalance, renal dysfunction, and respiratory distress syndrome.[2] Unstable patients, particularly those with cerebral malaria or significant respiratory sequelae, require intensive care.[2]
Treatment involves combination therapy targeting both the hepatic and erythrocytic forms.[17] The chief antimalarials are chloroquine, hydroxychloroquine, primaquine, artemisinin-based combination therapy (ACT), and atovaquone-proguanil. Chloroquine and hydroxychloroquine are synthetic forms of quinine.[18][19] They disrupt the erythrocytic stage by interfering with parasitic hemoglobin metabolism and increasing intracellular pH.[18][19] They generally require two days of treatment, allowing for better tolerance and shorter admissions.[2] However, chloroquine may enhance gametogenesis, contributing to resistance, which is a concern, particularly in South Asia.[17] Primaquine is a hypnozointocidal agent added for P. vivax or P. ovale infection for the eradication of liver parasites and the prevention of dormancy and relapse.[2][20]
Primaquine is contraindicated in pregnant and G6PD deficient patients due to fetal teratogenicity and hemolytic reaction (will see bite cells and Heinz bodies on blood smear), respectively.[3] Artemisinins are active against all parasite lifecycle stages.[2] Atovaquone targets the cellular electron transport chain inhibiting ATP production; proguanil enhances atovaquone’s effect by sensitizing parasitic mitochondria.[21] Atovaquone-proguanil is active against the erythrocytic and extraerythrocytic forms.[17][21]
Per the 2019 CDC Guidelines below, appropriate treatment depends on the Plasmodium species, clinical stability, age of the patient, and regional antimalarial susceptibility:
- Uncomplicated P. falciparum, P. malariae or P. knowlesi infections in chloroquine-sensitive regions are treated with a chloroquine phosphate 600 mg (pediatric: 10 mg/kg) loading dose, followed by 300 mg (pediatric: 5 mg/kg) at 6, 24, 48 hours; or a hydroxychloroquine 620 mg (pediatric: 10 mg/kg) loading dose, followed by 310 mg (pediatric: 5 mg/kg) at 6, 24, and 48 hours.
- Uncomplicated P. falciparum infections in chloroquine-resistant or unknown regions are treated with atovaquone-proguanil 250 mg/100 mg 4 tabs (pediatric: varied weight-based dosing, 6.5 mg/25 mg tabs) daily for 4 days; or artemether-lumefantrine 20 mg/120 mg 4 tabs (pediatric: varied weight-based tabs) at initial dose, then 8 hours later, then twice daily for 2 days; or quinine sulfate 542 mg (pediatric: 8.3 mg/kg) three times daily for 3 days (7 days if in Southeast Asia) plus either doxycycline 100 mg daily for 7 days (pediatrics 2.2 mg/kg every 12 hours), or tetracycline 250 mg daily for 7 days (pediatric: 25 mg/kg/day divided four times daily for 7 days), or clindamycin 20 mg/kg/day divided three times daily for 7 days (pediatric: same); or mefloquine 684 mg (pediatric: 13.7 mg/kg) loading dose followed by 456 mg (pediatric: 9.1 mg/kg) every 6 to 12 hours for total of 1250 mg (pediatric total: 25 mg/kg).
- Uncomplicated P. vivax or P. ovale infections in chloroquine-sensitive regions receive treatment with chloroquine phosphate or hydroxychloroquine as per above, plus either primaquine phosphate 30 mg (pediatric: 0.5 mg/kg) daily for 14 days or tafenoquine 300 mg once (same in children older than 16 years).
- Uncomplicated P. vivax infections in chloroquine-resistant regions (Indonesia, Papua New Guinea) get treated with quinine sulfate as per above plus either doxycycline, primaquine, or tafenoquine as per above; or atovaquone-proguanil as per above plus either primaquine or tafenoquine; or mefloquine as per above plus either primaquine or tafenoquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-sensitive regions require treatment with chloroquine or hydroxychloroquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-resistant regions are treated with quinine sulfate as per above plus either clindamycin or mefloquine as per above in the first, second, or third trimesters; or artemether-lumefantrine as per above in only the second and third trimesters.
- Severe malaria infection in unstable, non-pregnant patients in all regions includes IV artesunate 2.4 mg/kg (pediatric: children greater than 20 kg receive 2.4 mg/kg, children less than 20 kg receive 3.0 mg/kg) at 0, 12, 24, and 48 hours and either artemether-lumefantrine, atovaquone-proguanil, doxycycline, or mefloquine as per above.
Differential Diagnosis
The differential for undifferentiated fever is extremely broad and varies based on geographic location and age. In a 2017 review of fever in returning travelers, 77% had protozoal malaria, 18% had a bacterial enteric fever (Salmonella enterica, typhi, or paratyphi), and 5% had another infection. In patients presenting with fever and significant somnolence or seizures, viral or bacterial meningitis or meningoencephalitis must remain on the differential and prompt consideration of lumbar puncture.[2][22] Viral etiologies include avian influenza, Middle East respiratory syndrome coronavirus, hemorrhagic fever (Ebola virus, Lassa fever, Marburg hemorrhagic fever, Crimean-Congo hemorrhagic fever), yellow fever, dengue, Japanese encephalitis, Rift Valley fever, hepatitis virus (A or B), viral gastroenteritis, and rabies.[22] Bacterial etiologies include anthrax, epidemic typhus, ehrlichiosis, leptospirosis, melioidosis, murine (endemic) typhus, spotted fever group rickettsioses, Q fever, and Yersinia pestis.[22][2]
The differential in children varies by region, with the most likely etiology being a viral or bacterial infection. In a 2014 study of febrile children in a tropical region, 10.5% were diagnosed with malaria, 62% were diagnosed with a respiratory infection, 13.3% with a systemic bacterial infection (usually staphylococcus or streptococcus bacteremia), and 10.3% with gastroenteritis (viral or bacterial).[23] Urinary tract infection and typhoid may also be considerations. Meningitis must be ruled out in somnolent children.[23]
Treatment Planning
Table 1. Artemisinin combination therapy (ACT) regimens for treatment of uncomplicated Plasmodium falciparum malaria in nonpregnant adults and children
| Drugs |
Combinations |
Dose by body weight (kg) |
Notes |
| Artemether-lumefantrine[24] |
- Artemether 20 mg/ lumefantrine 120 mg
- Artemether 40 mg/ lumefantrine 240 mg
|
Weight Dose
- 5 to <15 20 mg/ 120 mg
- 15 to <25 40 mg/ 240 mg
- 25 to <35 60 mg/ 360 mg
- ≥35 80 mg/ 480 mg
Dose administered orally twice daily for 3 days
|
It should be taken after a full meal or whole milk. Repeat the dose if the patient vomits within 30 minutes of taking a dose. Ideally, the first two doses should be taken 8 hours apart. The efficacy may decline with an increase in body weight. Also, alternate agents may be considered for patients > 65 years. |
| Artesunate-mefloquine[25] |
- Artesunate 25 mg/ mefloquine 55 mg
- Artesunate 100 mg/ mefloquine 220 mg
|
Weight Dose
- 5 to <9 25 mg/ 55 mg
- 9 to <18 50 mg/ 110 mg
- 18 to <30 100 mg/ 220 mg
- ≥30 200 mg/ 440 mg
Dose administered orally twice daily for 3 days
|
Mefloquine is contraindicated for individuals with cardiac conduction abnormalities, neurologic, and psychiatric disorders. Also, avoid in patients with a significant family history of seizures and major psychiatric disorders.[26] |
| Artesunate-amodiaquine[27] |
- Artesunate 25 mg/ amodiaquine 67.5 mg
- Artesunate 50 mg/ amodiaquine 135 mg
- Artesunate 100 mg/ amodiaquine 270 mg
|
Weight Dose
- 4.5 to <9 25 mg/ 67.5 mg
- 9 to <18 50 mg/ 135 mg
- 18 to <36 100 mg/ 270 mg
- ≥36 200 mg/ 540 mg
Dose administered orally twice daily for 3 days
|
Amodiaquine is similar in structure to chloroquine; therefore, there is some cross-resistance between the two drugs.[28] |
| Artesunate-sulfadoxine-pyrimethamine |
- Co-packaged scored artesunate tablets (50 mg) and sulfadoxine-pyrimethamine (SP) tablets (500 mg sulfadoxine and 25 mg pyrimethamine)
|
Weight Artesunate (orally once daily for 3 days) SP (single dose orally on day 1)
- 5 to <10 25 mg 250 mg plus 12.5 mg
- 10 to <25 50 mg 500 mg plus 25 mg
- 25 to <50 100 mg 1000 mg plus 50 mg
- ≥50 200 mg 1500 mg plus 75 mg
|
Sulfadoxine-pyrimethamine can aggravate neonatal hyperbilirubinemia. Therefore, it should be avoided in the first weeks of life. |
| Artesunate-pyronaridine tetraphosphate |
- Artesunate 60 mg/ pyronaridine tetraphosphate 180 mg
- For children weighing ≤5 to 20 kg, as an oral suspension of granules from a sachet: Artesunate 20 mg/ pyronaridine tetraphosphate 60 mg
|
Weight Dose
- ≥5 to <8 20 mg/ 60 mg
- 8 to <15 40 mg/ 120 mg
- 15 to <20 60 mg/ 180 mg (use oral suspension)
- 20 to <24 60 mg/ 180 mg (use tablet)
- 24 to <45 120 mg/ 360 mg
- 45 to <65 180 mg/ 540 mg
- ≥65 240 mg/ 720 mg
Dose administered orally once daily for 3 days
|
Tablets and oral suspension can be taken with or without food. The required number of sachets is mixed with 10 mL of water in a small cup until the granules are suspended evenly and are then administered immediately. Repeat the dose if the patient vomits within 30 minutes of taking a dose. |
| Dihydroartemisinin-piperaquine (DP)[29] |
- Dihydroartemisinin 20 mg/ piperaquine 160 mg
- Dihydroartemisinin 40 mg/ piperaquine 320 mg
|
Weight Dose
- 5 to <8 20 mg/ 160 mg
- 8 to <11 30 mg/ 240 mg
- 11 to <17 40 mg/ 320 mg
- 17 to <25 60 mg/ 480 mg
- 25 to <36 80 mg/ 640 mg
- 36 to <60 120 mg/ 960 mg
- 60 to <80 160 mg/ 1280 mg
- ≥80 200 mg/ 1600 mg
Dose administered orally once daily for 3 days
|
DP may be taken with food but not with a high-fat meal. Also, it should not be used in patients with congenital QT prolongation or who are on medications that prolong the QT interval, as piperaquine prolongs QT interval (by the same amount as chloroquine but by less than quinine). |
Table 2. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women regions with chloroquine-resistant P. falciparum infection.
| Trimester |
Treatment regimen |
Dosages |
| First trimester |
Quinine PLUS clindamycin |
Quinine: 542 mg base (= 650 mg salt) three times daily for a weekClindamycin: 20 mg base/kg/day (up to 1.8 grams) divided three times daily for a weekORArtemisinin combination therapy can be used as an alternate therapy in the first trimester if the above treatment is unavailable or fails.
|
| Second or third trimester |
Artemisinin combination therapy |
Artemether-lumefantrine: 1 tablet = 20 mg artemether and 120 mg lumefantrine. A three-day regimen with a total of six oral doses is recommended as determined by the patient's weight.
- 25 to 34 kg: 3 tablets per dose
- ≥35 kg: 4 tablets per dose)[30]
Artesunate-amodiaquine: 1 tablet = 100 mg artesunate and 270 mg amodiaquine
- ≥36 kg: 2 tablets per day for three days
Artesunate-mefloquine: 1 tablet = 100 mg artesunate and 220 mg mefloquine hydrochloride per tablet.
- ≥30 kg: 2 tablets per day for three days
Dihydroartemisinin-piperaquine: 1 tablet = 40 mg of dihydroartemisinin and 320 mg of piperaquine phosphate.
- 36 to 60 kg: 3 tablets per day for three days
- 60 to 80 kg: 4 tablets per day for three days
|
Table 3. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women in regions with chloroquine-sensitive P. falciparum infection
| Drug |
Dosage |
| Chloroquine[31] |
- 00 hours: 600 mg base (= 1000 mg salt)
- 06 hours: 300 mg base (= 500 mg salt)
- 24 hours: 300 mg base (= 500 mg salt)
- 48 hours: 300 mg base (= 500 mg salt)
- Total dose: 1500 mg base (= 2500 mg salt)
|
| Hydroxychloroquine |
- 00 hours: 620 mg base (= 800 mg salt)
- 06 hours: 310 mg base (= 400 mg salt)
- 24 hours: 310 mg base (= 400 mg salt)
- 48 hours: 310 mg base (= 400 mg salt)
- Total dose: 1550 mg base (= 2000 mg salt)
|
Prognosis
The duration of untreated infection and time to relapse vary by location and species. P. falciparum and P. ovale infections last 2 to 3 weeks and may relapse 6 to 18 months later, usually from a new primary infection.[1] P. vivax infection lasts 3 to 8 weeks and may relapse months to up to 5 years later.[1] P. malariae infection lasts 3 to 24 weeks and may relapse up to 20 years later.[1]
Relapse is a case of recurrent symptoms months to years after the resolution of erythrocytic organisms due to reinfection or hypnozoite activation.[2][1] Recrudescence is defined as recurrent symptoms within days to weeks of acute illness due to remaining parasitemia after ineffective or incomplete treatment or failed host immune response, more commonly in P. falciparum.[2][1] Appropriate, complete treatment usually results in a full resolution of symptoms.
The two main determinants reflecting the outcome for both adults and children were the level of consciousness assessed by coma scales and the degree of metabolic acidosis, assessed clinically by breathing pattern or, more precisely, with measurement of bicarbonate, base deficit, and plasma lactate.[32] While the general mortality of treated severe malaria is between 10 to 20%, the mortality in pregnant women reaches approximately 50%.[14]
Complications
The significant complications of malaria are cerebral malaria, severe malarial anemia, and nephrotic syndrome (NS).
Cerebral malaria accounts for 80% of fatal malaria cases, most often occurring with P. falciparum infection.[1] It presents as slow-onset altered mental status, violent behavior, headache, and extremely high fever (up to 42 degrees C), followed by coma, metabolic acidosis, hypoglycemia, and possibly seizures and death.[1][4] It most commonly affects children under age 5, with a case fatality rate of 18%.[33] Pathogenesis involves malarial rosettes (one infected erythrocyte surrounded by three uninfected erythrocytes), causing cerebral sequestration and vasodilation, as well as excessive oxygen free radicals, IFN-gamma, and TNF-alpha leading to an extreme inflammatory response.[1][4][33] This leads to congestion, decreased perfusion, endothelial activation, impairment of the blood-brain barrier, and cerebral edema, which increases brain volume.[33]
Increased brain volume is the major contributor to mortality in cerebral malaria. In a 2015 study of Malawian children with cerebral malaria, 84% of those who died had severely increased brain volume on MRI; children who survived showed lower initial brain volume or a downtrend over time.[33]
Severe malarial anemia stems from TNF-alpha-mediated mechanisms involving both increased destruction and decreased production of erythrocytes, including cell lysis as parasites replicate and exit erythrocytes, splenic removal and autoimmune lysis of immune-marked erythrocytes, poor iron incorporation into new heme molecules, and bone marrow suppression during severe infection leading to decreased production.[1][4] Blackwater fever is severe anemia with hemoglobinuria and renal failure in the context of "massive intravascular hemolysis" in the setting of repeat P. falciparum infections treated with chronic quinine; it is rare and thought to be associated with G6PD deficiency.[34]
Nephrotic syndrome occurs secondary to glomerular antigen-antibody complex deposition and presents similarly to membranoproliferative glomerulonephritis with proteinuria and decreased renal function, which may lead to renal failure. Nephrotic syndrome is common in P. malariae and P. knowlesi, possible in P. vivax, and rare in P. falciparum and P. ovale infections.[1]
Additional complications include:
- Bilious remittent fever presents with abdominal pain and persistent vomiting that may lead to severe dehydration, jaundice, and dark urine.
- Algid malaria is an adrenal insufficiency due to parasitic congestion and subsequent necrosis of the adrenal glands.
- Acute respiratory distress syndrome, circulatory collapse, disseminated intravascular coagulation, pulmonary edema, coma, and death.[1]
Malaria infection during pregnancy may result in low birth weight or fetal demise.[1]
Consultations
Recommended consultations for non-infectious disease experts in the management or prevention of malaria include infectious disease and preventive or travel medicine.
Deterrence and Patient Education
The recommendation is that patients schedule a pre-travel appointment with a preventive medicine or infectious disease physician for education regarding malaria deterrence. Malaria prevention centers around vector control and chemoprophylaxis while exposed to mosquito-ridden environments.
Vector control is the prevention of mosquito bites by way of insecticide-impregnated bed nets, permethrin treatment of clothing, and DEET application to the skin.[3] The three main prophylactic agents for Plasmodium falciparum are atovaquone-proguanil, doxycycline, and mefloquine. Atovaquone-proguanil is taken once daily during and one week after travel to an endemic region; it suppresses the hepatic stage and does not have approval for pregnancy.[2] Doxycycline is taken once daily during and one month after travel; it suppresses the blood stage.[2] Doxycycline has the added benefit of prophylaxis against Rickettsial disease, Q fever, leptospirosis, and travelers’ diarrhea; however, it may cause gastrointestinal distress, photosensitivity, and increased risk of candida infection. Mefloquine is taken once weekly during and one month after travel; it suppresses the blood stage.[2] It has the benefit of safety in the second and third trimester of pregnancy; however, it has a far higher risk of neuropsychiatric side effects.[2] The US military primarily utilizes doxycycline if susceptibilities are equal.[2] For pregnant women in the first trimester or breastfeeding women, chloroquine or mefloquine prophylaxis are preferable; data regarding the safety of atovaquone-proguanil prophylaxis in pregnancy is limited.[35]
Enhancing Healthcare Team Outcomes
The timely care of patients diagnosed with malaria and clinically relevant research regarding advancing diagnostic techniques and treatment requires interprofessional teamwork and communication between clinicians, infectious disease experts, pharmacists, nurses, and global health professionals.
Any clinician treating malaria will initiate treatment as outlined above. Still, it is good policy to include an infectious disease specialist and involve an infectious disease board-certified pharmacist, who can also examine the regimen and agents chosen, as well as verify dosing and drug interactions. A nurse with infectious disease specialty training can also help by answering patient questions, serving as a bridge to the treating clinician, and monitoring treatment progress and potential adverse drug reactions. All team members must keep accurate and updated records, so everyone involved in treatment has the same information on the patient's case. If there are any concerns, each team member must be free to communicate with other team members so that appropriate interventions can be started or therapeutic modifications can be implemented. This collaborative interprofessional approach can optimize outcomes for malaria patients. [Level 5]