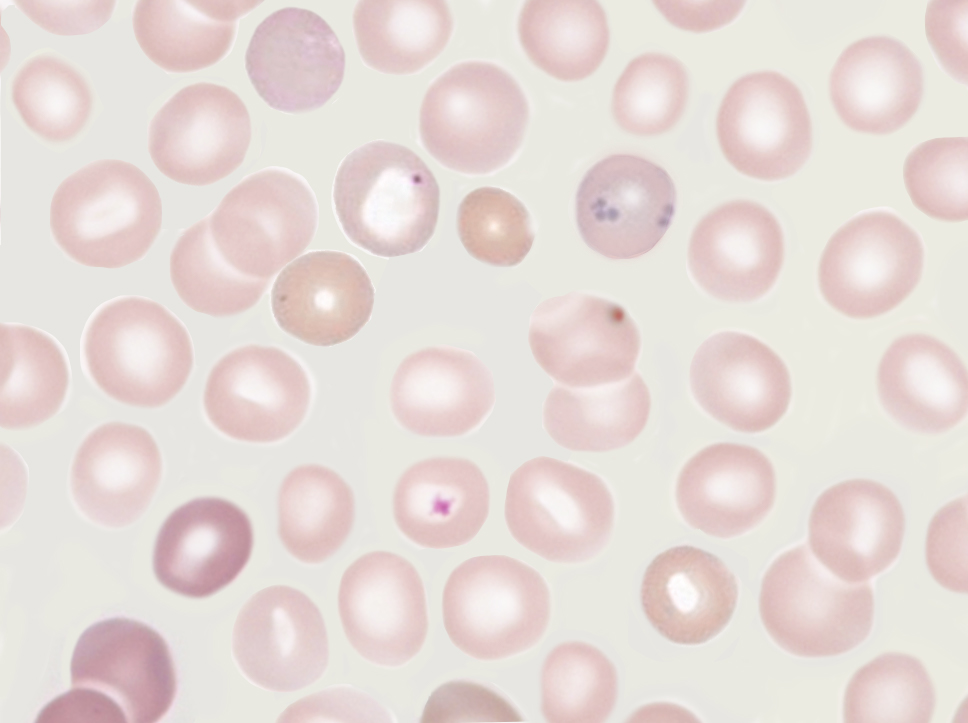
[1]
Sears DA, Udden MM. Howell-Jolly bodies: a brief historical review. The American journal of the medical sciences. 2012 May:343(5):407-9. doi: 10.1097/MAJ.0b013e31823020d1. Epub
[PubMed PMID: 21946828]
[2]
Ong SY, Ng HJ. Howell-Jolly bodies in systemic amyloidosis. International journal of hematology. 2018 Aug:108(2):119-120. doi: 10.1007/s12185-018-2473-8. Epub 2018 May 22
[PubMed PMID: 29790005]
[3]
William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology (Amsterdam, Netherlands). 2007 Feb:12(1):1-13
[PubMed PMID: 17364987]
[4]
Johns J, Goel AK, Jondhale S, Kumar Venkatesan D, Ravina M, Shah S, Syal S. Splenic Dysfunction in Children With Sickle Cell Disease: A Single Centre Experience From Central India. Indian pediatrics. 2024 Jun 20:():. pii: S097475591600654. Epub 2024 Jun 20
[PubMed PMID: 38910365]
[5]
Wagener MG, Marahrens H, Ganter M. Anaemia in South American camelids - an overview of clinical and laboratory diagnostics. Veterinary research communications. 2024 Apr:48(2):633-647. doi: 10.1007/s11259-023-10274-z. Epub 2023 Dec 5
[PubMed PMID: 38049672]
Level 3 (low-level) evidence
[6]
Narvestad-Bøttger H, Winther-Larsen A, Haugbølle Bjerre J, Dziegiel MH, Hansen AT, Hasle H. Extreme Reticulocytosis After Splenectomy in a Patient With Hemoglobin Mizuho. Journal of pediatric hematology/oncology. 2024 Jan 1:46(1):e111-e114. doi: 10.1097/MPH.0000000000002790. Epub 2023 Nov 24
[PubMed PMID: 38011049]
[7]
Butel-Simoes GI, Jones P, Wood EM, Spelman D, Woolley IJ, Ojaimi S. Congenital asplenia study: clinical and laboratory characterisation of adults with congenital asplenia. Annals of hematology. 2022 Jul:101(7):1421-1434. doi: 10.1007/s00277-022-04765-3. Epub 2022 Apr 22
[PubMed PMID: 35451619]
[8]
Scott-Charlton A, Reynolds G. A case of systemic lupus erythematosus associated auto-splenectomy presenting as invasive pneumococcal sepsis. Modern rheumatology case reports. 2020 Jul:4(2):233-236. doi: 10.1080/24725625.2020.1751407. Epub 2020 May 7
[PubMed PMID: 33087009]
Level 3 (low-level) evidence
[9]
de Porto AP, Lammers AJ, Bennink RJ, ten Berge IJ, Speelman P, Hoekstra JB. Assessment of splenic function. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010 Dec:29(12):1465-73. doi: 10.1007/s10096-010-1049-1. Epub 2010 Sep 19
[PubMed PMID: 20853172]
[10]
Felka T, Lemke J, Lemke C, Michel S, Liehr T, Claussen U. DNA degradation during maturation of erythrocytes - molecular cytogenetic characterization of Howell-Jolly bodies. Cytogenetic and genome research. 2007:119(1-2):2-8
[PubMed PMID: 18160774]
[11]
Yan X, Kong J, Wang J, Wang C, Shen H. Severe megaloblastic anemia in a patient with advanced lung adenocarcinoma during treatment with erlotinib: a case report and literature review. BMC pulmonary medicine. 2024 Mar 6:24(1):121. doi: 10.1186/s12890-024-02935-9. Epub 2024 Mar 6
[PubMed PMID: 38448889]
Level 3 (low-level) evidence
[12]
Harrod VL, Howard TA, Zimmerman SA, Dertinger SD, Ware RE. Quantitative analysis of Howell-Jolly bodies in children with sickle cell disease. Experimental hematology. 2007 Feb:35(2):179-83
[PubMed PMID: 17258066]
[13]
Mathew H, Dittus C, Malek A, Negroiu A. Howell-Jolly bodies on peripheral smear leading to the diagnosis of congenital hyposplenism in a patient with septic shock. Clinical case reports. 2015 Aug:3(8):714-7. doi: 10.1002/ccr3.323. Epub 2015 Jul 4
[PubMed PMID: 26331020]
Level 3 (low-level) evidence
[14]
Hilberg RW, Ringle RD, Balcerzak SP. Howell-Jolly bodies. Intracellular or extracellular? Archives of internal medicine. 1973 Feb:131(2):236-7
[PubMed PMID: 4682982]
[15]
JENSEN WN, MORENO GD, BESSIS MC. AN ELECTRON MICROSCOPIC DESCRIPTION OF BASOPHILIC STIPPLING IN RED CELLS. Blood. 1965 Jun:25():933-43
[PubMed PMID: 14294770]
[16]
RIFKIND RA, DANON D. HEINZ BODY ANEMIA--AN ULTRASTRUCTURAL STUDY. I. HEINZ BODY FORMATION. Blood. 1965 Jun:25():885-96
[PubMed PMID: 14294766]
[17]
Kashimura M. The human spleen as the center of the blood defense system. International journal of hematology. 2020 Aug:112(2):147-158. doi: 10.1007/s12185-020-02912-y. Epub 2020 Jun 16
[PubMed PMID: 32557229]
[18]
Nardo-Marino A, Braunstein TH, Petersen J, Brewin JN, Mottelson MN, Williams TN, Kurtzhals JAL, Rees DC, Glenthøj A. Automating Pitted Red Blood Cell Counts Using Deep Neural Network Analysis: A New Method for Measuring Splenic Function in Sickle Cell Anaemia. Frontiers in physiology. 2022:13():859906. doi: 10.3389/fphys.2022.859906. Epub 2022 Apr 5
[PubMed PMID: 35480040]
[19]
Angay O, Friedrich M, Pinnecker J, Hintzsche H, Stopper H, Hempel K, Heinze KG. Image-based modeling and scoring of Howell-Jolly Bodies in human erythrocytes. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2018 Mar:93(3):305-313. doi: 10.1002/cyto.a.23123. Epub 2017 May 24
[PubMed PMID: 28544333]
[20]
Araújo NC, Orlando MMC, Neves MB, Rioja SS, de Lucena SBG, Mandarim-de-Lacerda CA. Howell-Jolly bodies and liver-spleen scanning for assessment of splenic filtrative function yields discordant results in renal transplant recipients. Medicine. 2017 Dec:96(51):e9242. doi: 10.1097/MD.0000000000009242. Epub
[PubMed PMID: 29390481]
[21]
Pourdieu C, El Hoss S, Le Roux E, Pages J, Koehl B, Missud F, Holvoet L, Ithier G, Benkerrou M, Haouari Z, Da Costa L, El Nemer W, Laurance S, Aronovicz YC, Le Van Kim C, Fenneteau O, Lainey E, Brousse V. Relevance of Howell-Jolly body counts for measuring spleen function in sickle cell disease. American journal of hematology. 2023 May:98(5):E110-E112. doi: 10.1002/ajh.26879. Epub 2023 Feb 27
[PubMed PMID: 36794434]
[22]
Oehadian A, Huang I, Kartikasari A, Alisjahbana B, Prihatni D. Howell-Jolly Body-Like Inclusions in Coronavirus Disease 2019 (COVID-19): Possible Novel Findings. Journal of blood medicine. 2023:14():233-238. doi: 10.2147/JBM.S399596. Epub 2023 Mar 29
[PubMed PMID: 37016662]
[23]
Sharma P, Nampoothiri RV, Sharma P, Prakash G, Malhotra P, Varma N. Howell-Jolly Body-Like Inclusions in Neutrophils and Monocytes of a Transplant Recipient. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2018 Apr:34(2):381-382. doi: 10.1007/s12288-017-0865-1. Epub 2017 Aug 17
[PubMed PMID: 29622896]
[24]
Sears DA, Udden MM. Pappenheimer bodies: a brief historical review. American journal of hematology. 2004 Apr:75(4):249-50
[PubMed PMID: 15054821]
[25]
van Berkel M, Besselaar E, Kuijper P, Scharnhorst V. Instrument-dependent interference of Howell-Jolly bodies in reticulocyte enumeration. Clinical chemistry and laboratory medicine. 2013 Jun:51(6):e137-9. doi: 10.1515/cclm-2012-0790. Epub
[PubMed PMID: 23314550]