Continuing Education Activity
A hiatal hernia is a common condition characterized by the abnormal protrusion of the upper part of the stomach or other internal organs through the diaphragm's hiatus. A lax hiatus may lead to gastroesophageal reflux disease. The development of a hiatal hernia is multifactorial, with contributing factors including congenital anatomical defects, increased intraabdominal pressure (as seen in obesity, pregnancy, and chronic coughing), and age-related changes in the diaphragm's muscle tone.
Diagnosing a hiatal hernia typically involves a combination of clinical evaluation and imaging studies. Upper gastrointestinal endoscopy, barium swallow radiography, and esophageal manometry are commonly used to visualize the hernia, assess its size, and evaluate the associated esophageal motility disorders. Treatment options for hiatal hernia range from conservative to surgical approaches, depending on symptom severity and the presence of complications. Conservative measures include dietary modifications, weight loss, and pharmacological therapy to manage reflux symptoms. Surgical repair, such as laparoscopic Nissen fundoplication, is considered for individuals with severe or refractory symptoms or complications like esophagitis or Barrett esophagus.
This activity for healthcare professionals is designed to enhance learners' competence in evaluating and managing hiatal hernia. Participants gain a deeper understanding of this condition's pathogenesis, clinical presentation, and best diagnostic and management practices. Greater proficiency equips learners to collaborate effectively within an interprofessional team caring for patients with hiatal hernias, enhancing outcomes.
Objectives:
Identify the signs and symptoms indicative of a hiatal hernia.
Develop a clinically guided diagnostic plan for a suspected hiatal hernia case.
Implement an appropriate treatment approach for a patient diagnosed with a hiatal hernia.
Apply effective strategies to improve care coordination among interprofessional team members to facilitate positive outcomes for patients with hiatal hernias.
Introduction
A hiatal hernia is a medical condition characterized by the abnormal protrusion of the upper part of the stomach or other internal organs through the diaphragm's hiatus. The diaphragm is a muscular structure that assists in respiration. The diaphragm has a small opening, a hiatus, through which the esophagus passes before connecting to the stomach. The region where the esophagus joins the stomach is called the "gastroesophageal junction" (GEJ).
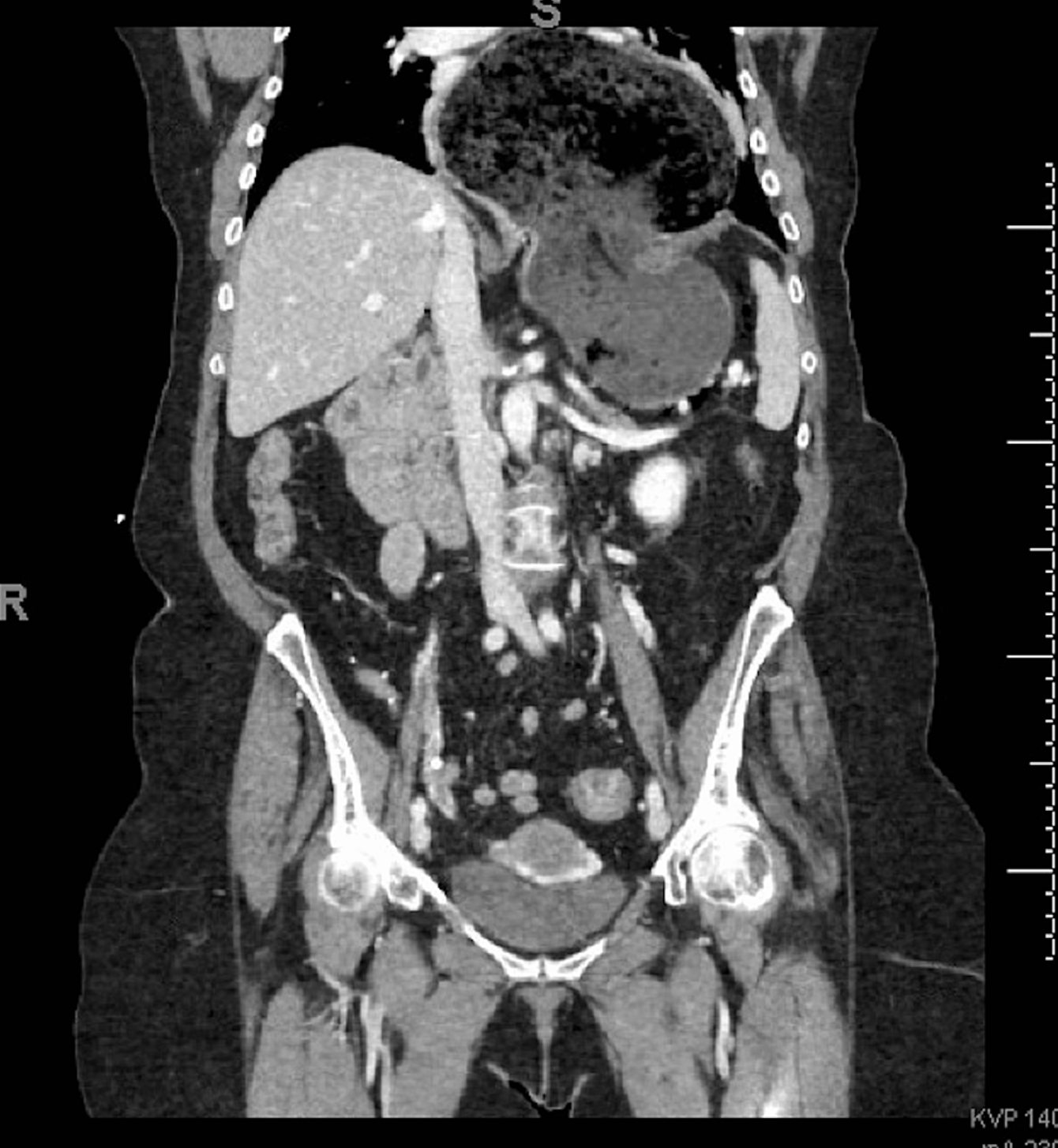
In a hiatal hernia, the stomach pushes through the diaphragmatic opening into the chest, compromising the lower esophageal sphincter (LES). This laxity of the LES may allow gastric contents and acid to back up into the esophagus, leading to gastroesophageal reflux disease (GERD). Small hiatal hernias are often asymptomatic and typically managed medically. Large hiatal hernias usually require surgery (see Image. Massive Hiatal Hernia).[1][2]
Hiatal hernias can cause symptoms such as heartburn, regurgitation, and difficulty swallowing. The condition is more common in adults than children and rarely results in life-threatening complications. However, severe hiatal hernias are typically repaired surgically through various procedures, often in conjunction with laparoscopic Nissen fundoplication.[3][4]
Hiatal hernias are classified into 4 types:
- Type I: The sliding type, representing more than 95% of hiatal hernias. This type occurs when the GEJ is displaced toward the hiatus.
- Type II: A paraesophageal hiatal hernia, which occurs when part of the stomach migrates into the mediastinum parallel to the esophagus
- Type III: A paraesophageal hernia combined with a sliding hernia, where both the GEJ and a portion of the stomach have migrated into the mediastinum
- Type IV: The stomach and an additional organ, such as the colon, small intestine, or spleen, herniate into the chest.[5][6]
Proper classification of this condition guides short- and long-term treatment.
Etiology
Hiatal hernias may be congenital or acquired. The condition's prevalence is increased among older people. Muscle weakness due to age-related loss of flexibility and elasticity is believed to be a predisposing factor to the development of a hiatal hernia. The hernia may prevent the upper part of the stomach from returning to its natural position below the diaphragm during swallowing. Other predisposing factors have been identified, such as elevated intraabdominal pressure, which typically arises from obesity, pregnancy, chronic constipation, and chronic obstructive pulmonary disease (COPD). Trauma, age, previous surgeries, and genetics also play a role in the development of a hiatal hernia.[7]
Epidemiology
The incidence of hiatal hernias increases with age. Approximately 55% to 60% of individuals older than 50 have a hiatal hernia. However, only about 9% have symptoms, with the manifestations depending on the type and competence of the LES. The vast majority of these hernias are type I sliding hiatal hernias. Type II paraesophageal hernias only comprise about 5% of hiatal hernias where the LES remains stationary, but the stomach protrudes above the diaphragm. The condition's prevalence is also increased in women, which may be attributed to elevated intraabdominal pressure during pregnancy. Hiatal hernias are most common in Western Europe and North America and are rare in rural Africa.[8]
History and Physical
GERD is the typical presentation leading to an evaluation for a hiatal hernia. Patients often complain of heartburn and, sometimes, regurgitation. While heartburn is the most common manifestation, some patients present with extraesophageal symptoms, such as a chronic cough or asthma. Regurgitation and extraesophageal symptoms usually signify disease progression. However, not all patients with regurgitation have GERD.
Whether the regurgitated food is digested or undigested must be noted. Undigested food may represent another pathology, such as achalasia or an esophageal diverticulum. Dysphagia is another problem seen in advanced disease, typically secondary to a mechanical obstruction. Dysphagia may signify the presence of an additional pathology such as a peptic stricture, tumor, diverticulum, or primary motor disorder.
Upper gastrointestinal symptoms, such as regurgitation, dysphagia, retention, and reflux, have traditionally been the primary focus when assessing the clinical impact of hiatal hernias. Most medical professionals do not commonly link hiatal hernias with chronic respiratory issues. However, earlier studies have indicated that preoperative respiratory symptoms are present in 30% to 44% of cases, suggesting that pulmonary problems in patients with hiatal hernia have often been overlooked. Patients with hiatal hernias are frequently older and usually have additional concurrent conditions that may contribute to respiratory problems.[9]
The physical examination in patients with a hiatal hernia and GERD rarely helps confirm the diagnosis. The presence of abnormal supraclavicular lymph nodes in patients with heartburn and dysphasia may suggest esophageal or gastric cancer and is an important part of the evaluation.[10][11]
Evaluation
The preoperative workup in a patient being considered for operative treatment will help confirm the diagnosis, exclude other pathologic entities, and direct the operative intervention. Endoscopy is an essential step in evaluating GERD and a suspected hiatal hernia in patients being considered for surgery. This study can exclude other diseases, such as tumors, and document the presence of esophageal injury. Manometry rules out primary motility disorders such as achalasia, which can mimic reflux symptoms. Patients with primary motility disorders often require a partial fundoplication rather than a Nissen.
The 24-hour pH test is the gold standard for diagnosing acid reflux. In this study, a probe is placed 5 cm above the GEJ and measures the amount of acid that passes in the region. The data obtained is then quantified using the DeMeester score, which is based on several parameters that include the amount of time esophageal pH is below 4, the number of reflux episodes, the duration of the longest reflux episode, and the total number of reflux episodes lasting longer than 5 minutes. A score of 14.7 or above indicates significant gastroesophageal reflux.[12] The esophagogram provides valuable information regarding the esophagus and proximal stomach anatomy. This study may also discover anatomic abnormalities such as tumors or strictures.
Treatment / Management
The management of hiatal hernias depends on the type of hernia and the severity of the symptoms. The initial treatment given to a patient presenting with typical GERD symptoms in an outpatient setting includes a double dose of a proton pump inhibitor (PPI), which can be both therapeutic and diagnostic in that persistent symptoms often require a more extensive evaluation.
The indications for surgical therapy have changed since the advent of PPIs. Individuals with evidence of severe esophageal injury, such as an ulcer, stricture, or Barrett mucosa, should be considered for surgical treatment. Other patients, such as those with a long duration or incomplete resolution of symptoms while on medical therapy, should also be considered for surgical intervention. The surgery cost has decreased with advancements in minimally invasive techniques for treating GERD. Surgical therapy may be considered the treatment of choice for patients with more than 8 years of life expectancy and who need lifelong therapy because of a mechanically defective LES.[13][14][15]
Paraesophageal hernias can present with a gastric volvulus due to the laxity of the stomach's peritoneal attachments and subsequent rotation of the gastric fundus. Such severe presentations are considered surgical emergencies. Current recommendations are for operative repair of all symptomatic paraesophageal hernias and completely asymptomatic large hernias in patients younger than 60 and otherwise healthy.[16]
The Nissen fundoplication (360° wrap) involves completely wrapping the GEJ using the stomach fundus. A 52-French bougie ensures approximation without the wrap being too tight. The initial steps involve the dissection of the short gastric vessels off the stomach's greater curvature to mobilize the fundus. The phrenoesophageal membrane over the left crus is thoroughly dissected, and the crural fibers are identified. The lesser omentum must be opened for the right crural dissection and the suitable phrenoesophageal membrane mobilized. The anterior and posterior vagus nerves must be preserved during dissection.
A Penrose drain is typically placed around the esophagus to assist in the mobilization and creation of the wrap, which is carried out over a 2.5 to 3 cm length using 3 to 4 interrupted permanent sutures. The 52-French bougie is removed once the wrap is complete. The wrap is anchored to the esophagus and hiatus to prevent herniation and slippage.[17]
A partial fundoplication is typically the procedure of choice when esophageal motility is poor. The 2 most common partial fundoplications are the Dor procedure, an anterior wrap, and the Toupet procedure, a posterior wrap. As opposed to the complete 360° wrap of the Nissen technique, these 2 procedures involve creating a 180° to 250° wrap. A partial wrap is thought to help prevent esophageal obstruction when motility is a concern.
The Dor procedure is performed by folding the fundus over the anterior aspect of the esophagus and then anchoring it to the hiatus and esophagus, as in the 360° wrap. This wrap has been used in a limited number of cases to treat GERD, particularly in patients with achalasia who have undergone an anterior myotomy.
Meanwhile, the Toupet procedure involves an entire esophageal dissection similar to that of a Nissen, with mobilization of the esophagus. However, as opposed to the Nissen, this procedure creates only a 220° to 250° wrap around the posterior aspect of the esophagus. The Toupet procedure is preferred if motility is a concern.[18]
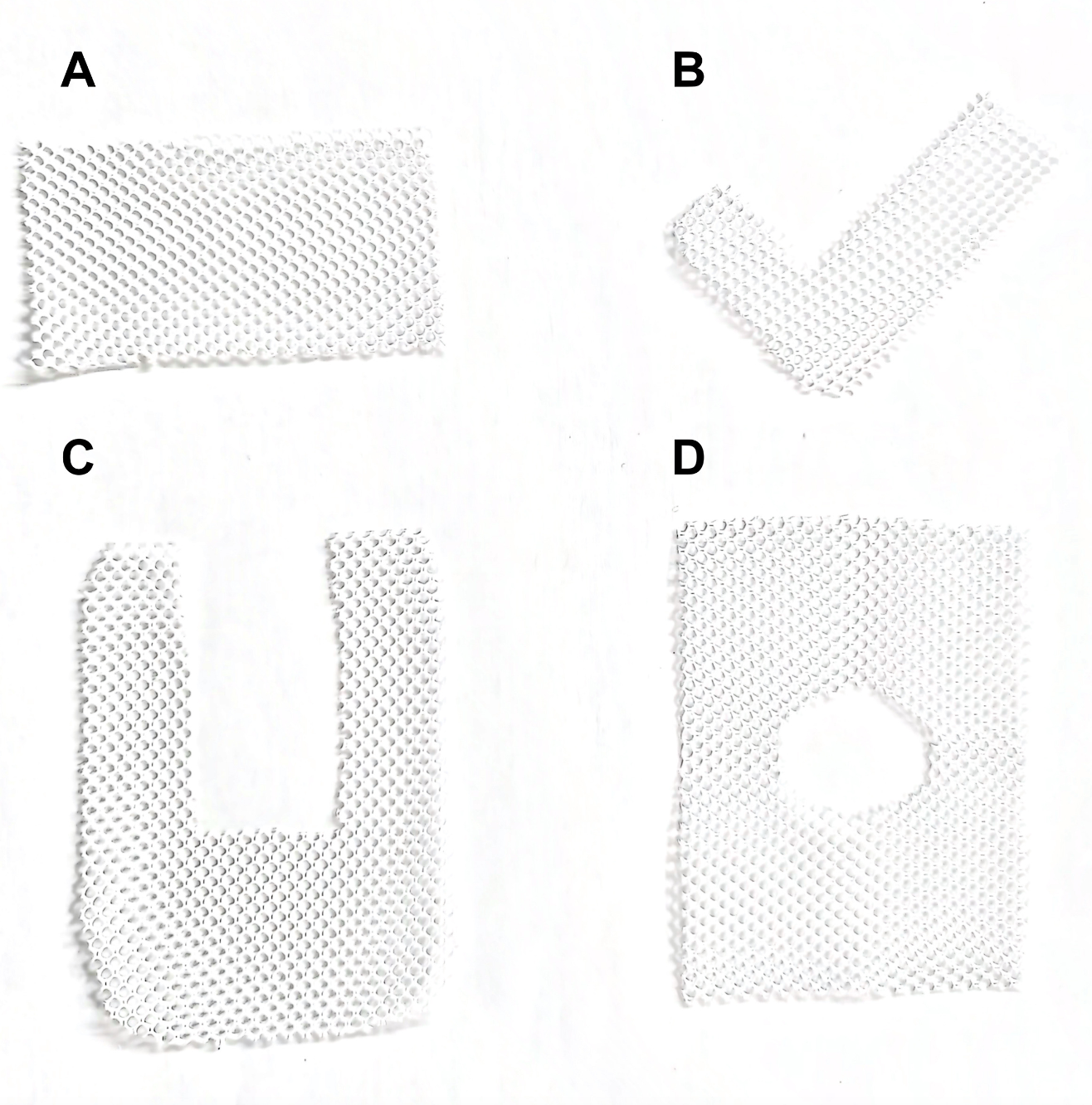
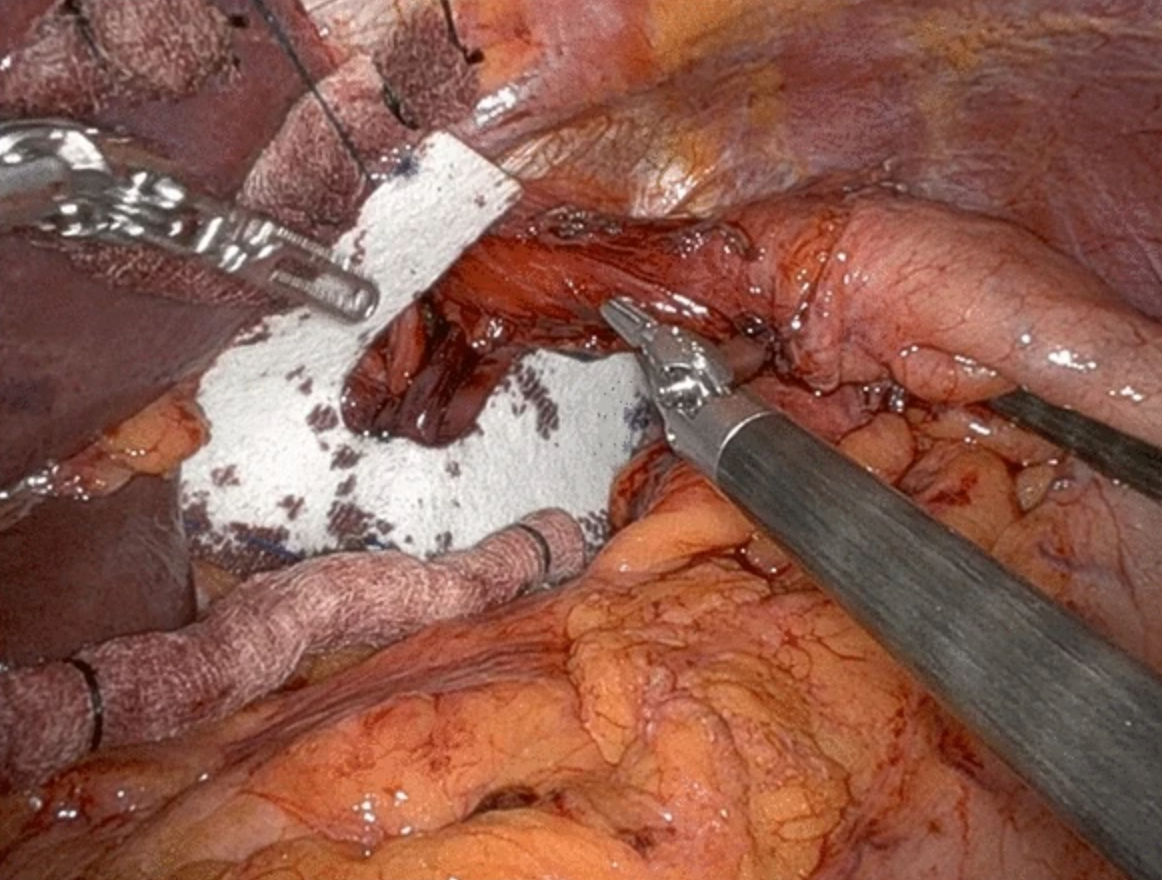
Advances in minimally invasive surgery have led to the widespread acceptance of laparoscopic hernia repair. Yet, a high recurrence rate remains despite this progress, particularly with large hernias repaired using primary sutures. To address this issue, mesh has become common in surgical practice. The mesh configurations used for hiatal hernias are varied (see Image. Mesh Configurations). Simple shapes like rectangular or triangular meshes were initially used to cover the primary closure. Over time, this approach evolved to include meshes designed with a central opening to facilitate the passage of the esophagus, such as U-shaped, keyhole, and A-shaped designs (see Image. Wrap and Mesh for Hiatal Hernia Surgery).[19]
Differential Diagnosis
The differential diagnosis of GERD can be quite extensive, thus the thorough workup before operative therapy. Typical heartburn is described as an epigastric caustic or burning sensation rather than a sensation of pressure that does not radiate to the back. This integral part of the history can distinguish GERD from pathologies such as acute coronary syndrome and pancreatitis.[20]
Extraesophageal GERD symptoms are often due to respiratory tract involvement, manifesting as laryngeal or pulmonary symptoms. Distinguishing the etiology of such symptoms can be challenging, requiring a high suspicion for primary esophageal motility disorders, gastric or esophageal cancer, and primary lung disease. Other etiologies must be explored if a patient presents with such symptoms and the primary workup does not support the initial diagnosis. A consultation with a pulmonologist is often warranted in such cases.[21]
Prognosis
The success of hiatal hernia surgery can be measured by evaluating symptom relief, improvement in esophageal acid exposure, the incidence of complications, and the need for reoperation. One prospective study followed 100 patients who underwent antireflux surgery over 10 years. A 90% reduction in symptoms was observed at 10 years. Operative management has continued to improve outcomes over the past 2 decades. Symptom improvement and perioperative complications have decreased with increased knowledge, especially in high-volume centers.[22]
A recent meta-analysis systematically reviewed the relationship between pulmonary function and hiatal hernia repair. This comprehensive analysis included 5 cohort studies with a total of 262 participants. Significant improvements in forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and total lung capacity (TLC) were observed following surgical repair. However, the residual volume and diffusing capacity of the lungs for carbon monoxide (DLCO) did not significantly get better. Still, these results indicate that surgical repair of hiatal hernias may benefit patients with associated pulmonary symptoms.
Complications
Data from a single institution previously showed that patients older than 72 with high frailty have double the risk of morbidity after elective paraesophageal hernia repair. This study mirrors findings on a national scale, indicating that age and frailty are significant predictors of morbidity after emergent hernia repair.[23][24]
Surgical complications are typically minor and not directly related to the surgery itself. The overall 30-day mortality rate associated with antireflux surgery is approximately 0.19%. Complications specific to antireflux surgery include pneumothorax, gastroesophageal or hepatosplenic injuries, and dysphagia. Pneumothorax is the most common intraoperative complication but is reported to occur in less than 2% of patients. Gastric or esophageal injuries occur in approximately 1% of patients undergoing Nissen fundoplication. Splenic and liver injuries can result in bleeding and occur in about 2.3% of patients. Major hepatosplenic injury is rare. Dysphagia typically resolves without further intervention and is most commonly caused by postoperative edema.[25]
Deterrence and Patient Education
Preventing a hiatal hernia involves measures that reduce intraabdominal pressure, such as maintaining a healthy weight, avoiding heavy lifting, and practicing good posture. Additionally, dividing meals into smaller portions taken more frequently and staying upright after eating can help minimize the risk.Patients receiving treatment for GERD and hiatal hernia-related symptoms should adhere to their medications and follow postoperative instructions closely. These instructions usually include modifying the diet in the postoperative period.
Enhancing Healthcare Team Outcomes
Managing hiatal hernias and reflux requires an interprofessional team approach. The team should include primary care clinicians, radiologists, gastroenterologists, and surgeons. Diagnostic studies are required for both diagnosis and operative planning. The workup typically includes endoscopy, pH monitoring, esophagography, and coordinated efforts between multiple teams. The interprofessional approach ultimately results in more accurate diagnoses, better surgical outcomes, and more patient satisfaction.
The enhanced availability of thoracic surgery services over the past 20 years supports this interprofessional approach. Most surgeons now report improved access to thoracic radiologists and medical oncologists, each at 81%. Thoracic surgeons also currently have access to thoracic radiation oncologists, pathologists, cytopathologists, respirologists, and gastroenterologists. Surgeons also receive support from dedicated dietitians/nutritionists, thoracic nurses, and physiotherapists. These collaborative services and resources align with the "practice setting" theme of Ontario Health and the Canadian Partnership Against Cancer standards, setting a benchmark for current thoracic surgery programs.