Continuing Education Activity
Traumatic brain injury (TBI) is an injury to the brain from an external mechanical force. TBI can generally be classified as either closed or penetrating, with the latter distinguished by a violation of the skull and dura mater. Of the two, closed head injury (CHI) is far more common. Types of CHI include concussion, contusion, diffuse axonal injury, and intracranial hematoma (epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraparenchymal hemorrhage). This activity will focus only on closed head injury, and the terms TBI and CHI will be used interchangeably. This activity describes the evaluation and management of closed head trauma and reviews the role of the interprofessional team in managing patients with this condition.
Objectives:
- Identify the etiology of closed head trauma emergencies.
- Outline the appropriate evaluation of closed head trauma.
- Review the treatment and management options available for closed head trauma.
- Describe the interprofessional team strategies for improving care coordination and communication to advance care for closed head trauma and improve outcomes.
Introduction
Traumatic brain injury (TBI) is an injury to the brain from external mechanical force. TBI can generally be classified as either closed or penetrating, with the latter distinguished by violation of the skull and dura mater. Of the two, closed head injury (CHI) is far more common. Types of CHI include concussion, contusion, diffuse axonal injury, and intracranial hematoma (epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraparenchymal hemorrhage). This article will focus only on closed head injury, and the terms TBI and CHI will be used interchangeably.
TBI is often classified as mild, moderate, or severe based on the Glasgow coma Scale (GCS). Mild TBI, often called “concussion,” is defined by a GCS of 14 to 15 and accounts for over 80% of TBI. Moderate TBI is defined by a GCS of 9 to 13 and accounts for about 10% of TBI. Severe TBI is defined by a GCS of 3 to 8.[1]
Etiology
The most common cause of CHI is a fall, accounting for over 35% of CHI, followed by motor vehicle/traffic injuries. Other causes include acts of violence, industrial injuries, and sports injuries. The possibility of non-accidental trauma, elder abuse, and domestic violence should be considered when evaluating a patient with CHI.[2]
Epidemiology
Across all age groups, CHI occurs more often in men than women. Falls cause over half of TBIs in children ages 0 to 14 years old and adults 65 years or older. Among children age 0 to 14, the second leading cause is being struck by or against an object, whereas the second leading cause among other age groups is motor vehicle/traffic injuries. Worldwide, TBI is responsible for greater than 30% of trauma deaths.[3][4]
Pathophysiology
Cerebral blood flow is technically difficult to determine. Cerebral perfusion pressure is the major factor in determining cerebral blood flow. Cerebral perfusion pressure (CPP) may be estimated by measuring intracranial pressure (ICP) and the mean arterial pressure (MAP), CPP=MAP-ICP. ICP itself is a function of the relative amounts of blood, brain parenchyma, and cerebrospinal fluid (CSF) in the inelastic space of the cranium. As put forth in the Monro-Kellie doctrine, an increase in the volume of blood, brain parenchyma, and/or CSF intracranially necessitates a compensatory decrease in the volume of another compartment or ICP will rise. As will be discussed below, head injury can increase ICP. If ICP approaches the MAP, CPP falls to dangerously low levels. The body responds by increasing the MAP and dilating cerebral blood vessels, which further raises ICP, thus continuing the vicious cycle that can lower CPP and lead to brain ischemia and long-term injury.
The factors that affect outcomes in CHI can be broken down into primary and secondary brain injuries. Primary brain injury is the direct damage to brain parenchyma from the force of impact. Primary brain injuries include contusions, hematomas, diffuse axonal injury (DAI), direct cellular damage, disruption to neurochemical and electrochemical function, and loss of the blood-brain barrier. Secondary brain injury is subsequent neuronal damage due to the release of neurotransmitters, the presence of inflammatory mediators, and apoptosis. Secondary brain injury should be distinguished from secondary insult, which refers to circumstances such as hypoxia and hypotension, among many others, that may accelerate brain damage and worsen outcomes.
Brain edema can occur extracellular swelling (cytotoxic edema), and this may be fatal by impairing cerebral perfusion and other mechanisms.[5][6][7][8]
History and Physical
Since many of these patients are often victims of multisystem trauma, ATLS (Advanced Trauma Life Support) protocol should be followed. History should be obtained from EMS, the patient (if able to provide a coherent history), family, and witnesses. It is vital to obtain history about the mechanism of injury, any focal neurologic deficits that were appreciated, seizures, vomiting, or changes in level of consciousness since the injury. Other important questions relate to intoxication, use of anticoagulant or antiplatelet medications, and other comorbid diseases.
An important element of both the history obtained from EMS and the physical examination is the determination of the Glasgow coma scale (GCS), as this will be trended throughout the hospital stay. GCS is scored on a scale of 1-15 based on eye-opening, verbal response, and motor response. On further physical exam of the head-injured patient with coma or altered mental status, a single fixed and dilated pupil is concerning for uncal herniation. Bilateral fixed and dilated pupils may indicate severely increased ICP with poor perfusion, bilateral uncal herniation, or hypoxemia. Assess movement in the upper and lower extremities in response to the command and/or noxious stimuli. Decorticate posturing (upper extremity flexion and lower extremity extension) indicates a severe injury likely above the level of the midbrain. Decerebrate posturing (extension and internal rotation of both the upper and lower extremities with wrist/finger flexion) indicates a more caudal brain injury. Examine brainstem reflexes (respiratory pattern, pupillary reflex, corneal reflex, cough, and gag reflex) in comatose patients who are useful for purposes of diagnosis and prognostication.
The physical exam should be repeated frequently, as changes may indicate increasing ICP or impending herniation. Signs and symptoms of increasing ICP include systolic hypertension, bradycardia, agonal respirations (these first three are collectively known as Cushing's triad), severe headache, vision changes, nausea, vomiting, lethargy, focal weakness/paresthesias, or coma. Signs of impending brain shift or herniation may include progressive neurologic decline, unilateral/bilateral pupillary dilation, hemiparesis, or abnormal posturing.
Evaluation
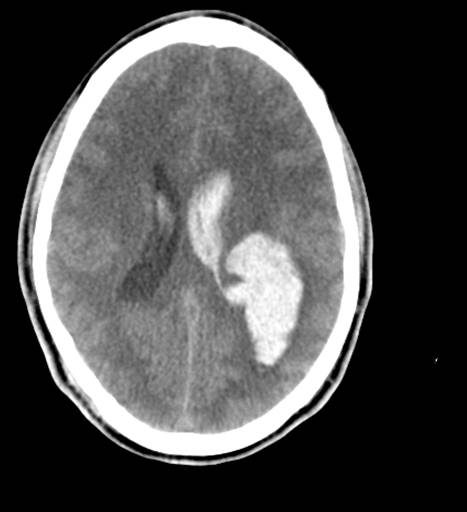
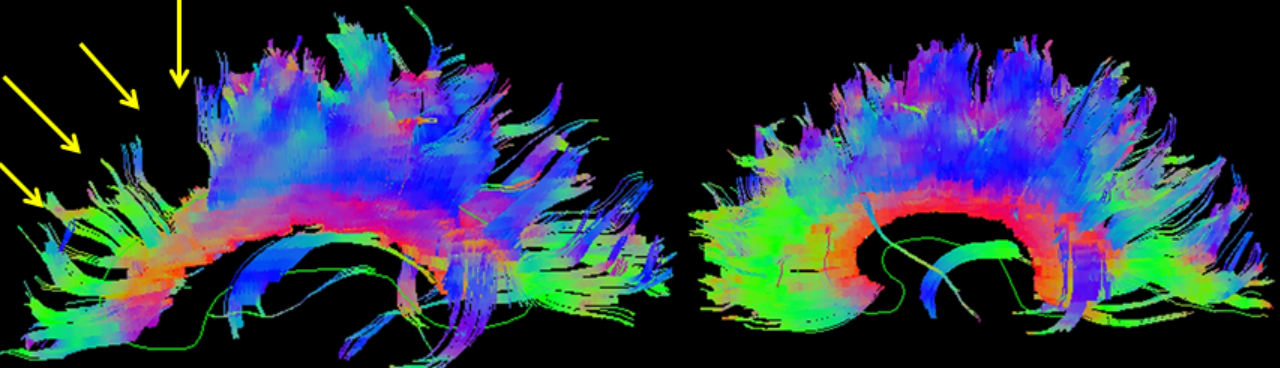
CT is exquisitely sensitive for acute intracranial bleeding. In a patient for whom there is concern for serious traumatic brain injury, CT should be obtained as rapidly as is safely possible. In an effort to minimize unnecessary CTs, there are several decision rules to aid in deciding which TBI patients need a head CT. These rules aim to identify patients who will require neurosurgical intervention. They do not identify patients who will have short or long term neurologic sequelae from TBI. The two most commonly utilized rules for adults are the Canadian Head CT Rule and the New Orleans Criteria. Both are 100% sensitive for clinically significant traumatic intracranial injuries, though the Canadian Head CT Rule is more specific. Notably, patients on antiplatelets and anticoagulants were excluded from these studies. For patients less than 18 years old, the most commonly used tool is the PECARN Pediatric Head Injury/Trauma Algorithm, which is also nearly 100% sensitive for clinically significant intracranial injuries. C-spine imaging should be strongly considered as well, especially in comatose patients. MRI may detect subtle lesions not picked up on CT or further define those seen on CT; however, as MRI may be difficult to obtain and has not been shown to be of significant value in the early evaluation of acute CHI, it is not a routine part of the initial evaluation. MRI may provide more information about chronic hemorrhage.[9][10][11]
Treatment / Management
Initial treatment should focus on the ABCs with the goal of maintaining cerebral perfusion and oxygenation.
Airway
TBI patients with a GCS less than or equal to 8 likely require intubation, as may those who are agitated to the extent of preventing necessary treatment. Perform rapid sequence intubation (RSI) using an induction agent with minimal effect on blood pressure or ICP, such as etomidate. The use of ketamine for induction remains controversial due to conflicting opinions regarding its effect on intracranial pressure, though recent evidence suggests it is safe to use in TBI. Succinylcholine and rocuronium are both safe and effective paralytic agents, though, with succinylcholine, the neuromuscular paralysis will resolve more quickly. Pretreatment with lidocaine or fentanyl was traditionally recommended for intubation of the neurotrauma patient. Though the evidence for fentanyl is better than that for lidocaine, neither has been shown to improve patient-centered outcomes. If facial trauma or basilar skull fracture is suspected, avoid nasotracheal intubation. It is recommended that in-line C-spine stabilization be maintained throughout intubation.[12][13]
Breathing
The goal should be normoxia and normocarbia, with a goal O2 sat >90%, PaO2 >60 mm Hg, and PaCO2 35 to 45 mm Hg. Hypoxemia is associated with a significant increase in mortality, and prolonged hypocapnia can cause cerebral vasoconstriction and subsequent ischemia.[14]
Circulation
The goal of circulation management is to maintain adequate CPP. As CPP = MAP - ICP, CPP is best preserved when MAP is high, and ICP is low. Though isolated head trauma is unlikely to cause hypotension, hypotension may be present secondary to bleeding from polytrauma, scalp lacerations, or subgaleal hematomas in children. Since hypotension may lead to brain ischemia and subsequent increases in edema, hypotension should be treated aggressively. Initiate fluid resuscitation to maintain systolic blood pressure at ≥100 mm Hg for patients 50 to 69 years old or at ≥110 mm Hg for patients 15 to 49 years old or >70 years old.[15][16][17]
Positioning
Though it is unclear whether elevating the head of the bed is clearly beneficial, elevation to 30 degrees is recommended in the setting of suspected increased ICP.[18]
Glucose
In patients with moderate to severe TBI, avoiding hyperglycemia is recommended. An insulin drip may be needed to maintain a goal of 100-180 mg/dL.[19]
Temperature
As fever can increase the metabolic demand of the brain, and may increase ICP, treat fever aggressively with a goal of normothermia. At this time, therapeutic hypothermia for TBI is not recommended.
Seizures
Since seizures are a common sequela of CHI and may worsen secondary injury, treat acute seizures with benzodiazepines. Seizure prophylaxis is more controversial but is recommended in patients with GCS <10, penetrating injury, depressed skull fracture, cortical contusion, intracranial hematoma, or seizure within the first 24 hours of head injury. Levetiracetam has shown to be as effective as phenytoin, but there is currently no recommendation as to the superiority of either agent to prevent seizures.[20][21]
Elevated ICP and Herniation
Early consultation with neurosurgery in the setting of moderate to severe TBI is recommended. Neurosurgery will help to direct surgical interventions and ICP assessment and monitoring with devices such as an intracranial bolt or external ventricular drain (EVD). A sustained ICP >20 mmHg is associated with increased morbidity and mortality, The Brain Trauma Foundation lists the following indications for invasive intracranial pressure monitoring: 1) Moderate to severe TBI in patients who cannot be accurately serially assessed by physical examination (for example intubated patients); 2) Severe head injury with abnormal CT scan; 3) Severe head injury with a normal CT if 2 of the following: age >40, systolic BP <90 mmHg, or abnormal motor posturing. If an intracranial bolt or EVD is not in place, herniation may be suspected clinically based on the signs and symptoms mentioned in the History and Physical section above. If there is concern for increasing ICP based on history, physical, or readings from an ICP monitor, mannitol and/or hypertonic saline can be used as treatment. Mannitol is an osmotic agent that can be delivered by repetitive boluses. As an osmotic diuretic, its use is relatively contraindicated in the presence of hemorrhage and hypotension, but it has also been shown to transiently reduce life-threatening elevations in ICP until other interventions may occur. Hypertonic saline, carried in most emergency departments in the form of 3% NaCl and in most ICUs in concentrations up to 23.4% NaCl, can be used as an alternative to mannitol, especially in those who are not adequately fluid resuscitated or have systemic hypotension.[22]
Differential Diagnosis
Consideration must be given to the event that led to the suspected injury. Dysrhythmias, cardiac ischemia, ischemic strokes, seizures, electrolyte abnormalities (most notably hypoglycemia), and toxic ingestions can cause a fall with CHI and mimic the altered mental status seen in CHI. Maintain high concern for concurrent orbital, maxillofacial, and skull trauma.
Prognosis
In the 2008 MRC CRASH study of 10,000 TBI patients with GCS <15, 1 in 5 patients were deceased at 2 weeks, 1 in 4 were deceased at 6 months, and 1 in 3 were deceased or severely disabled at 6 months. Clinical predictors of poor outcome include advanced age, initial post-resuscitation GCS, hypotension, hypoxia, pupil abnormalities, elevations in ICP, and co-morbid diseases. Radiological predictors of bad outcome include obliteration of the 3rd ventricle and/or basal cisterns, midline shift, petechial hemorrhage, subarachnoid hemorrhage, and brainstem injury. The two main tools used to predict outcome in TBI are the CRASH and IMPACT Head Injury Prognosis Calculators. The IMPACT prognosis calculator predicts a 6-month outcome in adult patients with moderate to severe TBI. The CRASH prognosis calculator predicts death at 14 days and death and severe disability at 6 months in adult patients with GCS 14 or less. Use caution when applying these results to individual patients since the outcomes apply at a population level.[23][24][25]
Special mention should be made of outcomes in mild traumatic brain injury, often called “concussion.” Adults and children less than 8 years old tend to recover quickly, with 85% to 90% recovering within 14 days. In adolescents and teenage males, recovery typically takes about 4 weeks, while teenage females may take longer than 4 weeks to recover. In contrast to older dogma, newer evidence has shown that early exercise leads to a quicker recovery.[26][27]
Complications
Complications and outcomes of moderate to severe TBI exist on a spectrum from good recovery (social participation or resumption of normal life with only minor deficits) to moderate disability (able to work but in a sheltered or reduced capacity) to severe disability (dependent on others for ADLs or self-caring but unable to work) to persistent vegetative state to death.
For mild TBI, potential sequelae include post-concussive syndrome (which is thought to occur in 80% of patients), second impact syndrome (a rare disorder that results in rapid cerebral edema and high mortality), post-traumatic epilepsy, and chronic traumatic encephalopathy, the likelihood of which increases with the number and frequency of concussions.
Deterrence and Patient Education
The best way to reduce morbidity and mortality in CHI is prevention. Prevention methods include seatbelt use, child safety seats or boosters, strict avoidance of motor vehicle operation under the influence of drugs or alcohol, helmet use when using open, unrestrained vehicles, helmet use during sports, fall prevention methods in the elderly and others at risk, and securing windows to prevent children from falling.
For patients with mild TBI, simple educational materials regarding return to activity and work/school have been shown to be effective in reducing symptoms. Many such materials are available online.
Enhancing Healthcare Team Outcomes
As severe TBI is a progressive and complex entity with significant morbidity and mortality, it is important that hospitals have protocols in place regarding initial and continuing evaluation and management of these patients. Such protocols should involve physicians, nurses, and other staff in the departments of the emergency department, trauma surgery, neurosurgery, and neurology. Pharmacists, case managers, social workers, physical and occupational therapists, and ethics teams may also have vital roles in both the inpatient and outpatient care of these patients, and they should be involved in treatment and care planning as early as possible.