Continuing Education Activity
Gestational trophoblastic disease (GTD) is a group of tumors defined by abnormal trophoblastic proliferation involving both benign and malignant entities. Histologically, GTD is divided into hydatidiform moles (containing villi) and other trophoblastic neoplasms (lacking villi). The nonmolar or malignant forms of GTD are called gestational trophoblastic neoplasia (GTN) and include the invasive mole, choriocarcinoma, epithelioid trophoblastic tumor (ETT), and placental-site trophoblastic tumor (PSTT). GTN can occur weeks or years following any pregnancy but occurs most commonly after a molar pregnancy. GTN can metastasize and be fatal if not treated. This activity reviews the causes and pathophysiology of GTD and highlights the role of the interprofessional team in its management.
Participants gain insights into evidence-based diagnostic approaches, including histopathological evaluation and imaging modalities, to facilitate accurate and timely diagnosis. Challenges in treatment, including considerations for fertility preservation, chemotherapy regimens, emerging targeted therapies, and follow-up surveillance, are addressed. Clinicians gain insight into patient-centered care and the need for effective communication and collaboration among the interprofessional team to achieve optimal, comprehensive care for patients with GTD.
Objectives:
Differentiate the pathophysiology and distinguishing features of various gestational trophoblastic disease subtypes, including hydatiform mole, invasive mole, choriocarcinoma, epithelioid trophoblastic tumor, and placental site trophoblastic tumor.
Implement screening methods and clinical assessment tools for diagnosing gestational trophoblastic disease.
Assess the current evidence-based treatment strategies for managing gestational trophoblastic disease, including surgical interventions, chemotherapy regimens, and targeted therapies.
Apply interprofessional team strategies for improving clinical coordination to ensure comprehensive and individualized care for patients with gestational trophoblastic disease.
Introduction
Gestational trophoblastic disease (GTD) is a group of tumors defined by abnormal trophoblastic proliferation.[1] Trophoblasts produce human chorionic gonadotropin (hCG). GTD is divided into hydatidiform moles (HM), which contain villi, and other trophoblastic neoplasms, which lack villi.[1] The nonmolar or malignant forms of GTD are called gestational trophoblastic neoplasia (GTN) and include the invasive mole, choriocarcinoma, epithelioid trophoblastic tumor (ETT), and placental site trophoblastic tumor (PSTT).[2] These malignancies can occur weeks or even years following any pregnancy but occur most commonly after a molar pregnancy.
The first description of GTD was by Hippocrates around 400 BC. However, it wasn't until 1895 that Felix Marchand discovered the association between pregnancy and GTD. When healthy trophoblastic tissue penetrates the endometrium, it creates the placenta, a rich uterine vasculature connecting the fetus and mother.
HM, or molar pregnancy, originates from the placenta. HM is categorized as a complete or partial mole and is usually considered the noninvasive form of GTD. Although HMs are usually considered benign, they are premalignant and can potentially become malignant and invasive.[3][4][5]
Although the behavior of a healthy trophoblast is well-controlled in benign GTD, the regulatory mechanisms can become dysfunctional, resulting in highly invasive vascular and metastatic tumors. GTN involves the malignant entities of GTD, including invasive mole, choriocarcinoma, ETT, and PSTT, all of which can metastasize and be fatal if not treated in a timely and effective manner.[6]
An invasive mole occurs when abnormal trophoblastic cells penetrate deeply into the uterine wall, potentially leading to complications. The rapid growth of abnormal tissue characterizes this condition and can result in abnormal uterine bleeding.
Choriocarcinoma is a rare and aggressive neoplasm.[7][1] The 2 significant choriocarcinoma subtypes, namely gestational and nongestational, have very different biological activity and prognoses. Choriocarcinoma predominately occurs in women but can also occur in men, usually as part of a mixed germ cell tumor.
ETT is a rare and distinctive subtype of GTN arising from the trophoblastic cells that contribute to the placenta during pregnancy. Characterized by its epithelioid cell morphology, ETT is known for its potential to present as an aggressive tumor. This variant often occurs in reproductive-aged women and can be associated with abnormal vaginal bleeding or a mass in the uterine cavity.
PSTT is another rare and distinctive form of GTN originating from the placental implantation site. Unlike more common trophoblastic tumors, PSTT tends to develop months or even years after a normal pregnancy, often following a term or molar gestation. This tumor is characterized by slow growth and can manifest with irregular vaginal bleeding.
Etiology
Trophoblasts, the first cells to differentiate from the fertilized ovum, supply the embryo with nutrients and eventually form the fetal portion of the placenta. There are 3 types of trophoblastic cells: cytotrophoblasts, syncytiotrophoblasts, and intermediate trophoblasts. The abnormal proliferation of these cells causes GTD. Transformations of cytotrophoblasts and syncytiotrophoblasts produce HMs and choriocarcinoma. Intermediate trophoblasts are associated with ETTs and PSTTs.[5]
HMs, or molar pregnancies, are benign trophoblastic tumors comprising approximately 80% of all GTD. Molar pregnancies are caused by abnormal gametogenesis and/or fertilization. These pregnancies are divided into complete and partial moles.
A complete molar pregnancy occurs when an enucleated egg is fertilized either by 2 sperm or by a haploid sperm, which then duplicates. Approximately 90% of complete moles have a 46,XX karyotype, while 10% have a 46,XY karyotype. Chromosomes of complete moles are paternally derived; however, the mitochondrial DNA is maternal.[5] Complete moles are the most common type of molar pregnancy, do not contain fetal parts, and tend to cause higher levels of hCG.
Partial molar pregnancies are generally triploid (69,XXX; 69,XXY; or 69,XYY) as a result of the fertilization of a haploid ovum by 2 sperms or a haploid sperm duplicating upon fertilization of a haploid ovum. Diploid karyotypes also can exist, resulting from the fertilization of an empty ovum by 2 sperm. Both maternal and paternal DNA is expressed in partial moles.[5] Partial moles may contain identifiable fetal parts.
Invasive moles specifically occur when these trophoblastic cells aggressively penetrate deeply into the uterine wall. The exact triggers for invasive mole development are not fully understood.
Choriocarcinoma develops from an abnormal trophoblastic population undergoing hyperplasia and anaplasia, most frequently following a molar pregnancy.[5] There are 2 forms of choriocarcinoma: gestational and nongestational. The former arises following an HM, normal pregnancy, or, most commonly, spontaneous abortion, while nongestational choriocarcinomas arise from pluripotent germ cells.[8] Nongestational choriocarcinomas form in males or females in the gonads or midline structures with pluripotent germ cells.[8]
The exact etiology of ETT remains somewhat elusive, given its rarity. ETT typically arises from trophoblastic cells involved in placental development during pregnancy. There is evidence suggesting a potential association with preceding pregnancies, particularly those with molar pregnancies or gestational trophoblastic disease.
PSTT is also rare and arises from the site of a previous pregnancy. While the precise triggers for the development of PSTT are not well-defined, it is believed to involve abnormal proliferation of trophoblastic cells at the implantation site of the placenta.
Epidemiology
Epidemiological studies have reported a significant variation in the incidence of HMs. Southeast Asia and Japan have the highest reported incidence, estimated at 2 in 1000 pregnancies. In the United States, HMs occur in approximately 1 in 600 therapeutic abortions and 1 in 1500 pregnancies. Differences in prevalence may reflect statistics based on population-based or hospital-based data. Proper management is essential as 15% to 20% of patients with HMs will develop malignant transformations requiring chemotherapy after surgical evacuation.[9]
Risk factors for molar pregnancy include extremes of age, ethnicity, and a prior history of an HM, suggesting a genetic etiology. The risk of a complete mole is higher for women older than 35 and younger than 21 years and 7.5 times higher for women older than 40. The risk of repeat molar pregnancy in women with a history of molar pregnancy is approximately 1%, which is 10 to 20 times the risk in the general population.[1] Dietary factors, including diets deficient in carotene (vitamin A precursor) and animal fats, and smoking also increase the risk of molar pregnancy.
Interestingly, a history of prior spontaneous abortion has been reported to confer a 2- to 3-fold increased risk of molar pregnancy compared to women without a history of spontaneous abortion.[5] Following 2 molar gestations, the risk of having a third mole is 15% to 20%.[10]
The true incidence of GTN is difficult to definitively quantify, as data concerning the total number of pregnancies and trophoblastic disease vary. Approximately 50% of GTN cases arise after a molar pregnancy, whereas 25% may develop after miscarriage, termination, or ectopic pregnancy; the remaining 25% may result after a preterm or term pregnancy.
When GTN develops after a molar pregnancy, it is usually an invasive mole or choriocarcinoma, rarely an ETT or a PSTT. After a complete molar pregnancy, approximately 15% of patients will have persistent local disease with invasion, and 5% may develop metastatic disease.
GTN after a nonmolar pregnancy occurs in about 2 to 200 per 100,000 pregnancies (depending on global reporting) and is typically a choriocarcinoma. GTN after pregnancy loss occurs in 1 in 15,000 cases; GTN after term pregnancy occurs in 1 in 150,000 cases.[11][12][13] The prevalence of choriocarcinomas, PSTTs, and ETTs is unclear, as these diseases can emerge following pregnancy.[6][14][13]
Choriocarcinoma is a very rare neoplasm with varied incidence worldwide.[1] Choriocarcinoma composes less than 0.1% of primary ovarian neoplasms in a pure form.[15] In the United States, choriocarcinoma occurs in approximately 1 in 20,000 to 40,000 pregnancies; 50% occur after term pregnancies, 25% after molar pregnancies, and 25% after other gestational events. In Europe, about 1 in 40,000 pregnant patients and 1 in 40 patients with HMs will develop choriocarcinoma.[16] In Southeast Asia and Japan, 9.2 in 40,000 pregnant women and 3.3 in 40 patients with HMs will subsequently develop choriocarcinoma.[1] In China, 1 in 2882 pregnant women will develop choriocarcinoma.[16] This correlates with an increased risk for the development of choriocarcinoma in Asian, Native American, and Black women. Other risk factors include prior complete HM (a 100-fold increased risk), advanced age, long-term oral contraceptive use, and blood type A.[1]
Choriocarcinoma can also occur in males, usually between the ages of 20 and 30.[17] Less than 1% of testicular tumors are pure choriocarcinoma.[15] Mixed germ cell tumors occur much more frequently in the testicle, with choriocarcinoma as a component in 15% of these tumors.[17]
In all populations, the incidence rates of both choriocarcinoma and HMs have declined over the past 30 years.[18][19]
Pathophysiology
The pathophysiology of HM involves the abnormal growth and development of placental tissue in the absence of a viable fetus. HMs are essentially very edematous immature placentas. A complete molar pregnancy results in the development of only placental parts, while a partial molar pregnancy also results in the development of some or all fetal parts.[20][21]
Both complete and partial moles are due to the over-proliferation of chorionic villi. It is still unclear if the trophoblastic transformation causes vascular obliteration and fetal death or, in another theory, a spontaneous stillbirth causes villous dysmorphism and trophoblastic proliferation. Several studies reveal a severe vasculogenic deficit in trophoblastic diseases, with significantly retarded angiogenesis in early complete mole, progressive accumulation of fluids, and subsequent formation of cystic spaces ("cistern").[22][23][24][25][26]
All types of GTD originate from the placenta. HMs and choriocarcinomas emerge from villous trophoblasts, whereas PSTTs and ETTs arise from intermediate trophoblasts. First-trimester complete HMs show aberrant budding, villous structures with trophoblastic hyperplasia, crumpled villous blood vessels, and stromal karyorrhectic debris. In contrast, early partial HMs show patchy villous hydrops, dispersed abnormally shaped erratic villi, patchy trophoblastic hyperplasia, and trophoblastic pseudo-inclusions.
Morphological differentiation of nonmolar miscarriage from a partial HM can be complicated, as villous dysmorphism can present without the characteristic trophoblastic hyperplasia of the partial mole. Furthermore, ploidy analysis demonstrated by in situ hybridization or flow cytometry can differentiate diploid from triploid conceptions. Still, it cannot distinguish between a complete mole and a diploid nonmolar miscarriage or molar and nonmolar triploid. In these instances, molecular investigations are necessary.
Infrequently, a molar pregnancy can coexist with a normal pregnancy. The diagnosis is usually made by ultrasound. Despite the high risk of spontaneous abortion, 40% to 60% of cases result in live births. The risk of GTN in a coexisting molar and normal pregnancy is 27% to 46%; in a singleton molar pregnancy, the risk is 15% to 20%. Pregnancy can proceed if there are no complications, negative genetic studies, and normal ultrasound findings.[27]
Choriocarcinomas are malignant hCG-releasing epithelial tumors with central necrosis and a characteristic biphasic structure. Intraplacental choriocarcinomas can also occur and are likely responsible for metastatic disease after term pregnancies. The majority of neonatal choriocarcinomas are a result of metastatic spread from intraplacental choriocarcinomas.
The exact pathogenesis of choriocarcinoma has not been fully explained or understood, but studies have shown cytotrophoblastic cells function as stem cells and undergo malignant transformation. The neoplastic cytotrophoblast further differentiates into intermediate trophoblasts and syncytiotrophoblasts.[28] The mixture of cells mimics the normal development of a pre-villous blastocyst, a feature seen in other gestational trophoblastic neoplasms.[29]
Overexpression of p53 and MDM2 has been demonstrated in choriocarcinoma, with no evidence of somatic mutation. Other genes implicated with either overexpression or down-regulation via hyper-methylation include NECC1, epidermal growth factor receptor, DOC-2/hDab2, Ras GTPase-activating protein, E-cadherin, HIC-1, p16, and TIMP3. HLA-G is demonstrated at very high levels in choriocarcinoma and functions to change the tumor microenvironment through the inactivation of the local immune system.[29]
ETTs, the rarest GTN types, demonstrate nests of tumor cells made of fairly uniform mononucleate intermediate trophoblasts. These nests are interspersed between necrotic debris and hyaline degeneration. ETTs may appear as discrete hemorrhagic, solid, or cystic lesions.[30][31]
PSTTs are malignant tumors formed from uterine lesions with less hemorrhage, necrosis, and lower hCG concentrations than choriocarcinomas. Human placental lactogen (hPL), expressed by syncytiotrophoblasts, is highly expressed in the trophoblasts of PSTTs.[32]
Extremely rare cases of mixed GTN tumors have been reported. Choriocarcinoma may coexist with PSTT and ETT. Diagnosis and treatment of these neoplasms is challenging due to limited data.[33]
Histopathology
Histological Features of Gestational Trophoblastic Disease Types
Complete Hydatidiform Moles
Karyotype: 46,XX (majority); 46,XY; no fetus/embryo, diffuse swelling of villi, and expanded trophoblastic hyperplasia.
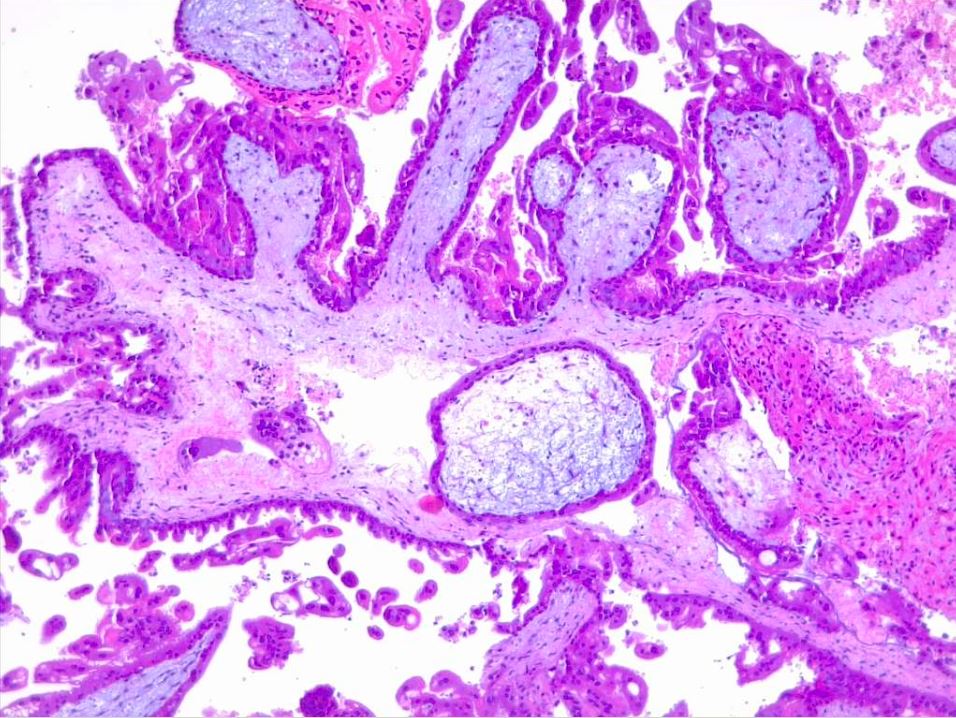
Hydatiform mole is characterized by overgrown villous trophoblasts with cystic "swollen" villi. Macroscopically, this can be visible during the second trimester as clusters of vesicles (similar to small grapes) develop from the transformation of chorionic villi.
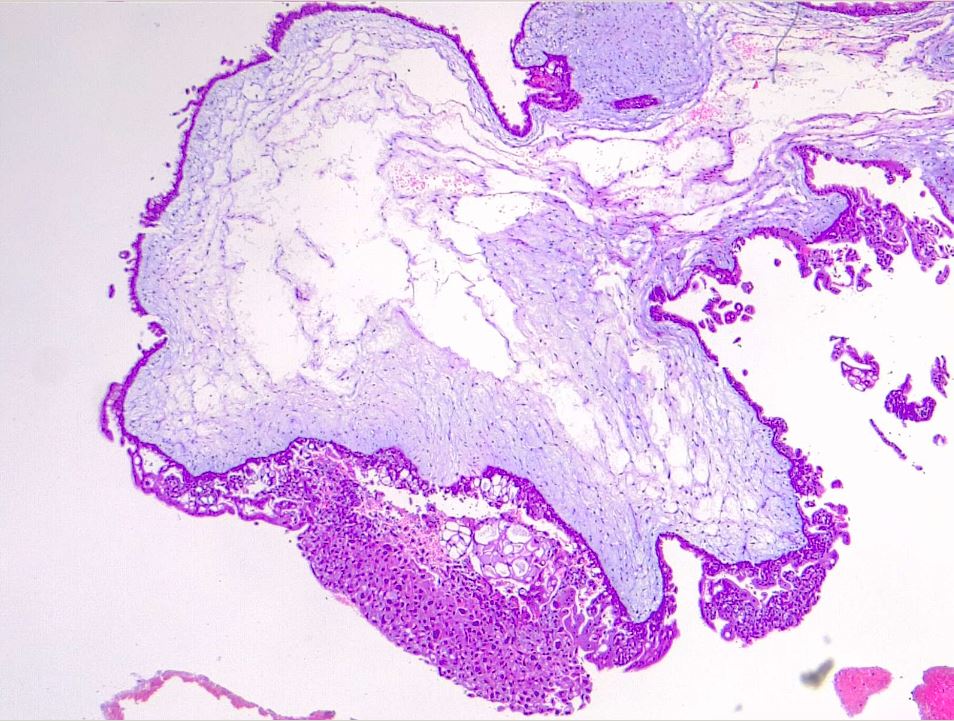
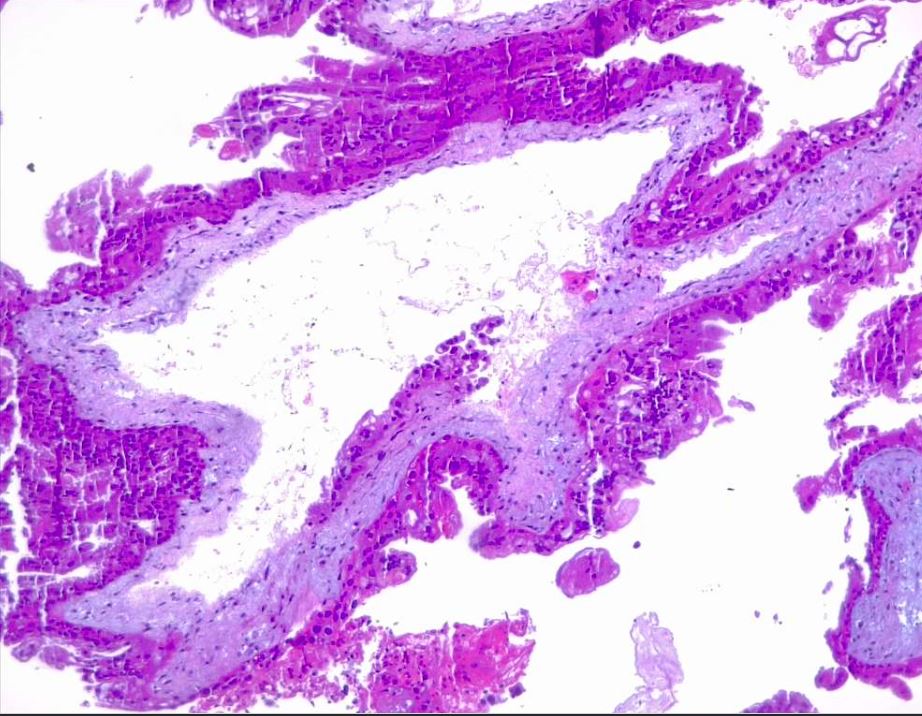
Microscopically, a complete mole has markedly hydropic and deformed chorionic villi, forming "cisterns" containing stromal fluid (see Image. Hydatiform Mole). A peripheral proliferation of both cytotrophoblasts and syncytiotrophoblasts is arranged in lace-like, papillary, and/or circumferential structures (see Image. Hydropic Villi and Trophoblastic Proliferation). In normal early placentas, the cytotrophoblasts and syncytiotrophoblasts are polarized. Fetal tissue and villous capillaries are absent in complete moles.[20][21] An immature vascular network is otherwise present, positive for CD31, with dysmorphic features such as a complete lack of lumen. There is almost always some degree of cytologic atypia, with many cells being mitotically active (see Image. Dysmorphic Villi in Hydatiform Mole). Central cisterns and trophoblastic hyperplasia are also present. Stromal changes such as stromal mucin and apoptosis appear early and can aid diagnosis.
An important marker that aids in diagnosing the complete mole is the presence or absence of p57 in immunohistochemistry. This marker is a paternal-imprint inhibitor gene, so its expression implies the maternal contribution; in brief, the absence of p57 expression supports the diagnosis of androgenetic gestational disease with a complete mole.[34][35][36] Moles also express p53, p21, cyclin E, and MCM7.
The primary differential diagnosis is with a hydropic abortion, where the main clue is the presence of villous edema only with microscopic evaluation and lack of cistern formation or trophoblastic proliferation.
Partial Hydatidiform Moles
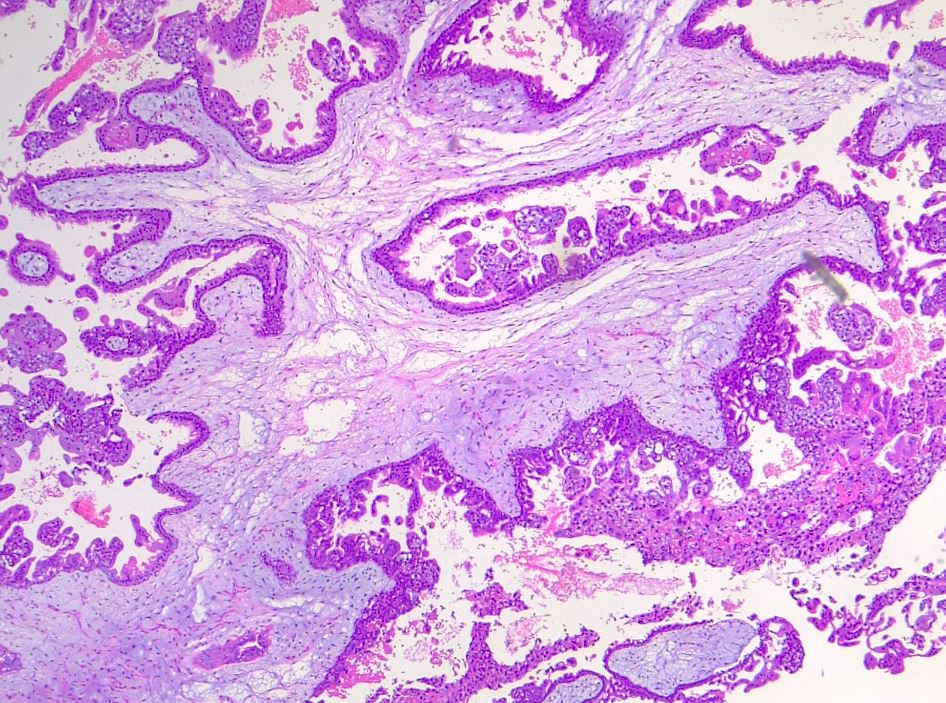
Karyotype: triploid (69,XXY; 69,XYY; 69,XXX); abnormal fetus/embryo, central swelling of villi, and central trophoblastic hyperplasia
In partial moles, there are hydropic chorionic villi surrounded by hyperplastic trophoblasts with variable degrees of central cistern formation with an irregular maze-like pattern. Also, there are normal chorionic villi and embryonic or fetal tissue mixed with hydropic villi (see Image. Irregular, Indented Villi with Lace-Like Trophoblast Proliferation). There are recognizable fetal blood vessels containing fetal red blood cells. The curettage material should be examined carefully, especially in first-trimester pregnancies, and if a partial mole is suspected, the whole specimen should be examined.
Choriocarcinoma
Diffusely penetrative growth involving the endomyometrium is present. Tumor cells recapitulate chorionic villous trophoblasts of different types and are arranged in biphasic to triphasic growth arrangements. Sheets or cords of mononuclear tumor cells are surrounded by layers of multinuclear syncytiotrophoblasts. Lymphovascular tumor thrombi are usually present.
Epithelioid Trophoblastic Tumor
This tumor usually creates discrete nodules or cystic hemorrhagic masses that deeply invade the surrounding tissues. Hemorrhage, necrosis, ulceration, and fistulas can be seen. Characteristically, ETT demonstrates nodular, expandable growth of uniform, medium-sized tumor cells organized in nests, cords, or large sheets.[1][37]
Placental Site Trophoblastic Tumor
Tumor cells invade the myometrium with vascular/lymphatic invasion. Focal hemorrhage and necrosis are seen in nearly one-half of cases, whereas transmural myometrial invasion is seen in 10% of cases. Perforation can occur.
History and Physical
HMs are most often diagnosed during the first trimester of pregnancy. The most prominent symptom (in 1 study, as high as 84% of patients) of a molar pregnancy is heavy vaginal bleeding early in the pregnancy, which is usually due to the molar tissue separating from the decidua.[38] The blood may be dark brown or prune juice-like in color. This is secondary to the accumulated blood products in the uterine cavity and the resultant oxidation and liquefaction of that blood. Some patients pass vaginal tissue resembling grape-like clusters or vesicles.[39] Other symptoms include hyperemesis (severe nausea and vomiting), which is due to the high level of the hCG hormone circulating in the bloodstream, and pregnancy-induced hypertension, often observed in the first trimester.
Later symptoms (after the first trimester, around 14 to 16 weeks of pregnancy) include signs and symptoms of hyperthyroidism, including tachycardia and tremors, again caused by the high levels of circulating hCG. Other late sequelae are pre-eclampsia, which is pregnancy-induced hypertension, and proteinuria and/or end-organ dysfunction, occurring typically after 34 weeks of pregnancy.[40][41] When a patient before 20 weeks of pregnancy presents with signs and symptoms of pre-eclampsia, a complete molar pregnancy should be highly suspected. Much later in pregnancy and rarely, patients may present with severe respiratory distress, possibly secondary to a pulmonary embolization of trophoblastic tissue.[42] Patients may also be asymptomatic at the time of diagnosis.[43]
The presentation of a partial HM is typically less dramatic than that of a complete HM. Patients with a partial mole may present with symptoms of vaginal bleeding similar to a threatened or spontaneous abortion, but most are typically not diagnosed before surgical uterine evacuation. These patients may also present with the diagnosis of a missed abortion.[44] Since partial HMs have fetal tissue, on examination, these patients may have fetal heart tones evident on Doppler. Histological examination of curettage specimens after incomplete or missed abortion is required for the diagnosis.
During a physical exam, in more than 50% of cases, there is a discrepancy between uterine size and dates. In a complete mole, the uterus is usually larger than the expected gestational date of the pregnancy, whereas, in partial moles, the uterus can be smaller than the suggested date. Adnexal enlargement due to theca lutein cysts may be present on pelvic examination, and fetal heart tones will be absent in patients with complete moles.[39]
The healthcare professional should conduct a thorough history and physical examination for any patient with suspected choriocarcinoma. Clinicians should pay particular attention to reproductive history because spontaneous abortions and molar pregnancies increase the risk for choriocarcinoma. One should consider post-menopausal bleeding suspicious. Choriocarcinoma tends to metastasize, and clinicians should note symptoms that arise from other organ systems, for example, hemoptysis or gastrointestinal (GI) bleeding.[16]
Males can present with gynecomastia and/or symptoms of metastatic disease, often hemoptysis, but the liver, GI tract, and brain are also frequently involved.[15][17] In males who have developed choriocarcinoma, the testicular anatomy is usually very small or even regressed, leaving only metastatic disease and cells.[17]
Evaluation
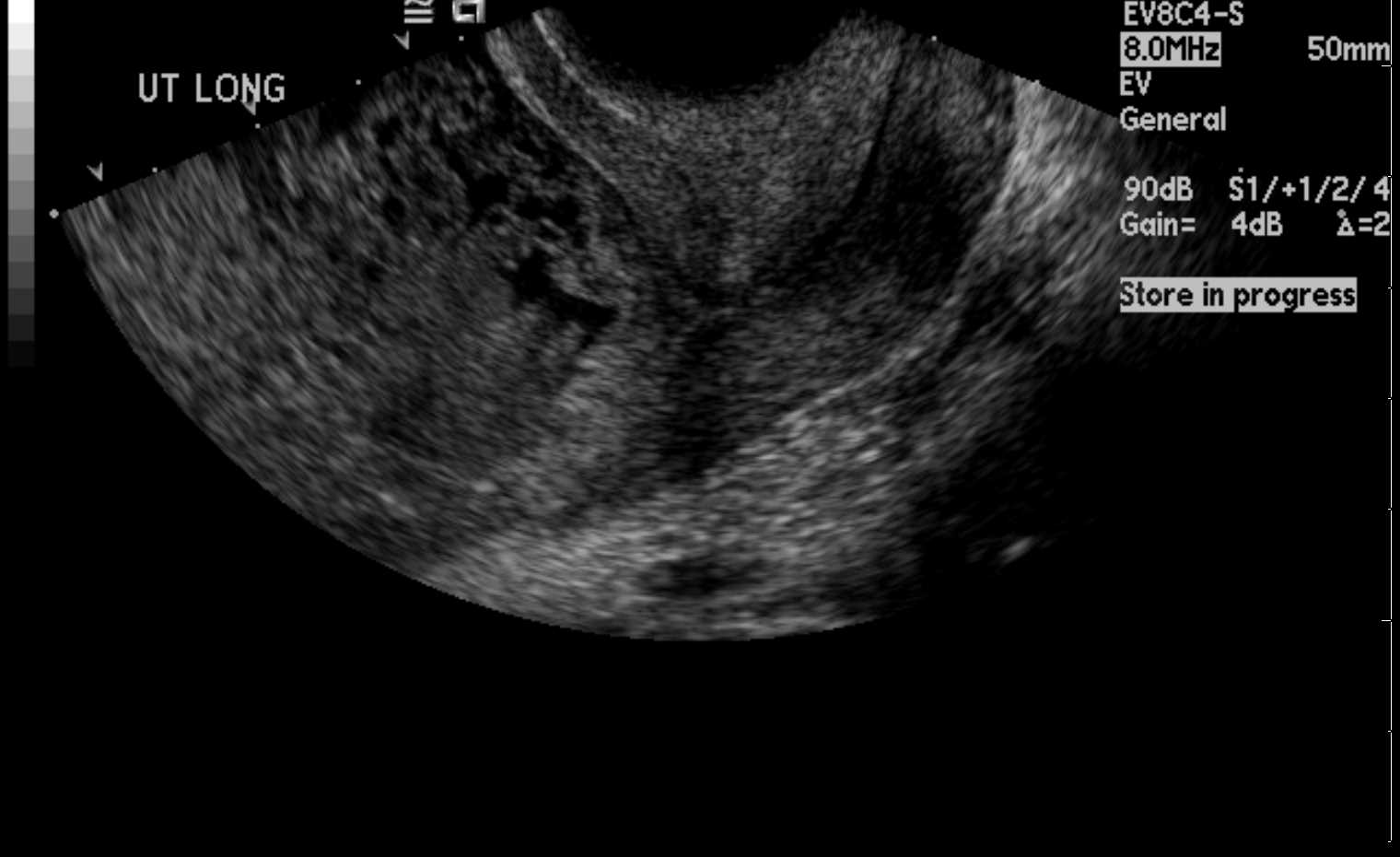
Ultrasound is the primary diagnostic tool for GTD.[45] The classically described appearance of a molar pregnancy on ultrasound is the uterus's "snowstorm" or "bunch of grapes" pattern (see Image. Uterus Ultrasound, Molar Pregnancy).[46] There is an absence of fetal development and amniotic fluid and, often, the presence of theca lutein ovarian cysts. However, this is less frequently seen today due to earlier diagnoses in the first trimester. Most first-trimester complete moles have a sonographic appearance of a complex, echogenic intrauterine mass containing multiple small cystic spaces. These spaces correspond to the hydropic villi on gross pathology.[46]
Despite ultrasound's diagnostic utility, in patients presumed to have had a spontaneous abortion, a molar pregnancy is detected only after pathologic evaluation. This most often also occurs with a partial molar pregnancy. In other instances of partial moles, ultrasound may reveal fetal components or a viable fetus, amniotic fluid, and an enlarged, thickened placenta (often described as having a "Swiss cheese" appearance).[5][46] Partial moles are often diagnosed as a missed or incomplete abortion.
Due to high false-negative and false-positive rates with ultrasound, specifically in the case of partial HMs, histological examination is necessary to reach the final diagnosis. As histological examination may not be practical after every termination, testing the hCG level 3 to 4 weeks post-termination to verify a negative result is highly recommended.[6]
If a molar pregnancy is diagnosed, the next step is typically a CT scan and PET scan to stage the disease. Furthermore, a chest x-ray should be obtained if the patient's initial symptoms include any signs of respiratory distress or increased breathing to evaluate for pulmonary edema.[47][21][48]
Routine preoperative evaluation of GTD includes the following:
- Quantitative human chorionic gonadotropin (hCG) level
- CBC with differential and platelet count (to evaluate for anemia and thrombocytopenia)
- Clotting studies (including PT/INR, to evaluate for disseminated intravascular coagulation in severe cases)
- Basic metabolic panel (to evaluate for electrolyte imbalance and renal insufficiency)
- Liver function tests (to evaluate for preeclampsia and/or transaminitis)
- Thyroid function tests (if signs and symptoms of hyperthyroidism present)
- Blood type with antibody screen
- Urinalysis (to evaluate for proteinuria)
- Chest x-ray [49]
Blood type is critical as most patients with complete and partial HMs present with vaginal bleeding. Rh antibody screening is required to determine the need for anti-D immunoglobulin administration in Rh(D) negative patients.[49]
In patients with complete HMs, the serum hCG levels are typically much higher than in patients of the same gestational date in a normal pregnancy or ectopic pregnancy. In contrast, partial moles may be within the normal range for gestational age or even lower than expected. Nearly one-half of patients with complete HMs have pre-evacuation hCG levels >100,000 mIU/mL. Such elevated levels occur in less than 10% of patients with partial HMs.[6]
The United Kingdom registers and monitors all patients with hydatidiform or molar pregnancies and utilizes the following criteria to start chemotherapy for GTD: [6]
- Plateaued or rising hCG following uterine evacuation
- Heavy vaginal bleeding
- Gastrointestinal or intraperitoneal bleeding
- Histologic evidence of choriocarcinoma
- Evidence of metastases in brain, liver, or gastrointestinal tract
- Lung opacities >2 cm
- Serum hCG >20,000 mIU/mL 4 weeks following evacuation
- Elevated hCG for longer than 6 months after evacuation, even when decreasing
Patients with postmolar GTN often present without symptoms or characteristic ultrasound findings; therefore, a correlation between hCG levels and tumor burden is necessary to reach the diagnosis. Many laboratory tests can assess for choriocarcinoma, including CBC, coagulation studies, body chemistries, renal function panels, liver function panels, type and screen, and quantitative hCG.[50]
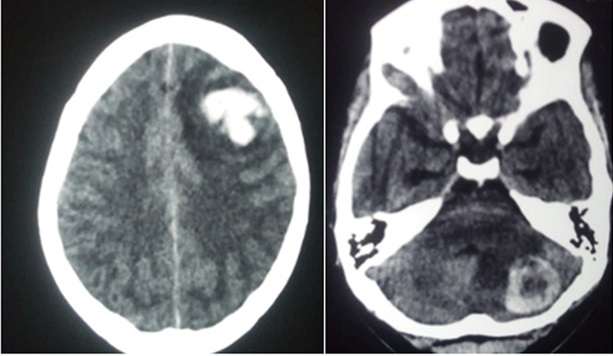
Following the diagnosis of choriocarcinoma, the healthcare professional should evaluate patients for metastasis; the lungs are the most common site for metastasis.[16] Chest, abdomen, and pelvic computed tomography are recommended in staging due to the highly metastatic nature of choriocarcinoma. The brain should be evaluated via computer tomography or magnetic resonance imaging (MRI) (see Image. Choriocarcinoma Metastasis to Brain).[50] MRI is essential in the routine evaluation of cases with atypical presentations, recurrence, ETT, or PSTT.[45]
Treatment / Management
If molar pregnancy is suspected from ultrasound findings and hCG levels, evaluation should be undertaken for possible medical complications, such as electrolyte imbalances from hyperemesis, anemia, hyperthyroidism, and preeclampsia. After evaluation, the decision on the method of evacuation is necessary. Medical induction and hysterotomy are contraindicated due to the risk of hemorrhage.
Surgical uterine evacuation (dilation and evacuation [D&E], suction curettage) is the mainstay of management for complete and partial moles regardless of uterine size.[51] Ultrasound guidance may be helpful for large uteri to ensure complete evacuation.[44] Rho (D) immunoglobulin should be administered after the procedure if the patient is Rh-negative.
Hysterectomy is an option for patients who have completed childbearing, particularly women of advanced maternal age. Hysterectomy reduces the risks of developing GTN, which is >50% in women older than 40 years, compared to suction evacuation and can eliminate the need for subsequent chemotherapy. Hysterectomy may, therefore, be particularly reasonable for patients older than 40 years with complete HMs.[52][53] There is some concern that inducing uterine contractions with uterotonics may increase the risk of metastatic disease.[1][51]
Prophylactic chemotherapy at the evacuation of high-risk complete HMs may reduce the incidence of GTN. When there is a significant concern about the reliability or availability of hCG follow-up, some clinicians consider chemoprophylaxis in patients with high-risk complete HMs.[44] However, a recent Cochrane review does not recommend this practice.[54]
All patients with molar pregnancies should be monitored with serial serum hCG values after evacuation to evaluate for post-molar GTN. Guidelines from the American College of Obstetricians and Gynecologists (ACOG) advise the following hCG monitoring protocol:
- Every week until non-detectable for 3 weeks, then
- Every month for 6 months
- If the hCG remains undetectable for 6 months, the patient may resume attempting to conceive.[44]
Following the evacuation of a complete or partial molar pregnancy, if hCG levels rise or remain elevated over several weeks, the patient is classified as having GTN. The diagnosis of post-molar GTN is based upon the International Federation of Gynecology and Obstetrics (FIGO) criteria:
- hCG levels plateau (remain within ±10% of the previous result) across 4 measurements over a 3-week period
- hCG level increases >10% across 3 values over a 2-week duration
- Persistence of detectable serum hCG for longer than 6 months after molar evacuation[2][39]
A low-risk (cumulative score <7; see staging section below) and stage I to III choriocarcinoma can be treated with a single agent, either methotrexate or actinomycin D chemotherapy.
High-risk (a cumulative score >7; see staging section below) and stage II to IV disease are treated with multi-agent chemotherapy, adjuvant radiation, and surgery.[50]
Following treatment and hCG normalization, quantitative hCG levels should be checked monthly for 1 year with a physical exam twice in the same time frame. If a subsequent pregnancy occurs, a first-trimester pelvic ultrasound should be performed to confirm a uterine location due to the small but present risk of recurrent choriocarcinoma; the placenta should be submitted for histologic examination of recurrence.[50]
Standard Gestational Trophoblastic Disease Treatments
These treatments include D&E, chemotherapy, hysterectomy, or a combination of these modalities.[50] Different treatment modalities are available for GTN, depending on the type and stage.
D&E: D&E is more commonly performed in molar pregnancies where fertility is desired. Monitoring after D&E is essential to ensure no disease recurrence.
Chemotherapy: Certain types of GTN can be treated by single or combination chemotherapy. Some chemotherapeutic agents used include methotrexate, etoposide, actinomycin D, cyclophosphamide, cisplatin, and vincristine.
Hysterectomy: A hysterectomy is the most common treatment option in cases of chemoresistance, severe disease, or lack of desire for future fertility.[5]
Initial management of nonmetastatic PSTT or ETT is hysterectomy and salpingectomy, with or without lymph node sampling. In metastatic PSTT or ETT cases, if feasible, hysterectomy, salpingectomy, and resection of metastatic disease are followed by platinum-based chemotherapy. [NCCN Guidelines 2022]
Controversial Treatments
Prophylactic chemotherapy: Prophylactic chemotherapy has been proposed in place of monitoring hCG levels until disease clearance criteria are met. The treatment has been reported to decrease intense chemotherapy regimens and boost the chance of complete healing.[5][54] However, a recent Cochrane Review discourages this practice.[54][55]
Second D&E: A second D&E may be performed after a molar pregnancy evacuation if the hCG level remains nonreassuring. However, if there is a high risk of uterine perforation or hemorrhage, a second D&E must not be performed.[5]
A prospective, multicenter study was conducted in Canada in 2016 to evaluate the efficacy and safety of this procedure in 64 patients with low-risk, nonmetastatic GTN, using the World Health Organization's (WHO's) prognostic score. The study concluded that a second D&E cured 40% of the patients without complications when used as an initial treatment for low-risk, nonmetastatic GTN.[55]
Drug-resistant GTN: Approximately 0.5% to 5% of patients experience drug-resistant GTN and may die. Pembrolizumab is one of the drugs studied for use in this situation, and the results show that it represents a valuable new approach for treating drug-resistant GTN.[56] Other more complex regimens have been proposed, yet were found to be associated with additional adverse effects. Future randomized controlled trials must be conducted to assess alternate regimens and their efficacy.[57]
Stabilization and Consultations
In the emergency department, the priority is clinical stabilization. If there is any evidence of respiratory distress and pulmonary edema, non-invasive positive pressure ventilation (such as BiPAP) or mechanical ventilation should be started. Furthermore, if there are signs or symptoms of eclampsia (late stage of preeclampsia), including seizures, one should initiate appropriate management, including benzodiazepines and magnesium sulfate administration. If the patient has signs of preeclampsia, urgent blood pressure control is necessary with medications such as hydralazine and labetalol. If there are signs and symptoms of hyperthyroidism, clinicians should initiate proper treatment, including beta-blockers, and monitor for thyroid storm. If severe anemia is present, the clinician should consider initiating a blood transfusion. As discussed earlier, if a patient is Rh(D) negative, the physician should administer the anti-D immunoglobulin.
Once the patient is stabilized, an emergent obstetrics consultation is necessary for the likely need for D&E. In patients with advanced maternal age, typically older than 40 years, and those who have completed childbearing, a hysterectomy is often performed instead of a D&E. Hysterectomy, however, does not entirely eliminate the risk of metastatic disease. After the evacuation of the molar pregnancy, the hCG levels should be monitored; if the levels remain elevated, there is evidence of persistent or invasive disease requiring, at times, chemotherapy. A gynecological oncologist consultation is usually necessary to guide therapy in these cases.
Differential Diagnosis
The differential diagnosis for GTD includes the following:
- Ectopic pregnancy
- Normal pregnancy
- Cornual pregnancy
- Pregnancy in the rudimentary uterine horn
- Missed abortion
- Threatened abortion
- Incomplete abortion
- Complete abortion
- Hydropic abortion
- Blighted ovum
- hCG-secreting germ cell tumor
- Quiescent GTN
- Mosaic conception
- Placental mesenchymal dysplasia
- Other placental abnormalities
- Uterine fibroids
- Ovarian cysts
- Ovarian tumors
- Hyperemesis gravidarum
- Hypertension
- Malignant hypertension
- Hyperthyroidism
- Biliary obstruction
- Bladder cancer [1][17][21][58][59]
Medical Oncology
In patients with postmolar GTN, evaluation involves a detailed history, physical exam including a pelvic exam, pelvic ultrasound, and imaging including a chest x-ray and/or chest, abdominal, and pelvic computed tomography (CT) scans. If no extrauterine disease is present, single-agent therapy is appropriate. Single-agent therapy for nonmetastatic disease is generally methotrexate or dactinomycin.
With extrauterine disease, the FIGO stage and prognostic score should be determined to assess if the patient is at a low or high risk for persistent or recurrent disease. Studies have reported a 2.9% recurrence rate in patients with nonmetastatic disease and up to a 9.1% recurrence rate in patients with metastatic disease.[59]
Scoring for metastatic disease is used to determine treatment. In low-risk metastatic disease (prognostic score <7), single-agent methotrexate or dactinomycin is appropriate. Between 10% and 30% of patients with low-risk GTN will develop resistance after single-agent chemotherapy, whereas up to 50% of patients with high-risk metastatic disease will develop resistance. Patients who develop resistance to the initial single agent usually will respond to an alternative single agent, and only 5% to 10% of patients will require multiagent therapy.[60][61][62]
Multiagent chemotherapy is indicated in high-risk metastatic disease (prognostic score ≥7), and guidelines recommend etoposide, methotrexate, and actinomycin D alternating with cyclophosphamide and vincristine (EMA/CO). However, up to 40% of patients with high-risk metastatic GTN may not respond or may have a relapse. There are no evidence-based guidelines for second-line treatment, although platinum-based regimens are generally utilized.[63]
Staging
GTN staging is based on tumor location and extent as follows:
- Stage I disease is confined to the uterus
- Stage II disease involves direct extension or metastasis to other genital structures
- Stage III disease is identified by lung metastasis
- Stage IV disease includes nonpulmonary distant metastasis
There are 3 primary systems used to stage and score GTD:
- The Clinical Classification System by the National Institute of Health (NIH)
- The WHO Prognostic Scoring System
- The FIGO Staging and Risk Factor Scoring System, which was revised and edited in 2000 [59][64]
The Clinical Classification System is widely used in the United States. This approach originated from the analyses of patients with metastatic GTN treated at the NIH. The system differentiates patients based on the presence or absence of metastatic disease, as all patients with nonmetastatic disease can be cured with initial single-agent chemotherapy.
Patients with metastatic disease are further subdivided according to the presence or absence of factors associated with response to initial single-agent chemotherapy. Patients with no high-risk clinical factors will likely benefit from initial single-agent therapy and are labeled as having good-prognosis metastatic GTD; conversely, patients with a single high-risk clinical factor are labeled as having poor-prognosis metastatic GTD. These patients with poor prognosis are at increased risk of failing single-agent chemotherapy and dying if treated with single-agent therapy followed by multiagent regimens.
The WHO Prognostic Scoring System offers precise information regarding disease prognosis. A 97% correlation of risk categorization was shown between the original WHO (1983) and FIGO 2000 systems.[65] The current FIGO prognostic scoring system was adapted from the WHO classification. FIGO prognostic scoring is based on individual risk factors that have been shown to predict GTN resistance to single-agent chemotherapy (see Table. FIGO Prognostic Scoring System).
Table. FIGO Prognostic Scoring System
| Score |
0 |
1 |
2 |
4 |
| Prognostic factor |
|
|
|
|
| Age (yr) |
<40 |
≥40 |
_ |
_ |
| Antecedent pregnancy |
Molar pregnancy |
Abortion |
Term pregnancy |
_ |
| Interval from index pregnancy (mo) |
<4 |
4-6 |
7-12 |
>12 |
| Pretreatment hCG (mIU/mL) |
<1000 |
1000-10,000
|
10,000-100,000
|
≥100,000 |
| Largest tumor size, including uterus (cm) |
<3 |
3-5 |
>5 |
_ |
| Sites of metastases |
Lung |
Spleen, kidney |
Gastrointestinal |
Brain, liver |
| Number of metastases |
0 |
1-4 |
5-8 |
>8 |
|
Previously failed chemotherapy
|
None |
None |
Single drug |
2 or more drugs |
[Modified from NCCN guidelines Gestational Trophoblastic Disease 2022]
The patient's diagnosis is assigned to a stage group represented by a Roman numeral: I, II, III, and IV. Next, the sum of the individual prognostic scores is noted after a colon. For example, stage II:4 or stage IV:9. Each patient with GTD will be given a stage and score.
For patients with PSTT or ETT, only the stage will be given. A risk factor score is not applicable in these cases.
Low-risk GTD has a total prognostic score of <7.
High-risk GTD has a total prognostic score of ≥7.[65][66][49]
Prognosis
Overall, the prognosis for GTD is very good. Patients with localized low-risk GTD are cured and have excellent survival rates. The prognosis for patients with localized high-risk and metastatic, low-risk GTD is also very good. Both with or without surgery, even high-risk metastatic GTD cure rates are 80% to 90% with a combination of chemotherapy and radiation.[67][68]
Low-Risk Disease
More than 80% of HMs are benign. The risk of invasive disease in a complete molar pregnancy is approximately 15% to 20%, and in a partial molar pregnancy is 1% to 5%.[1] About 95% of patients diagnosed with an HM who develop neoplasia have a low risk of persistence. For most patients, single-agent chemotherapy with methotrexate or dactinomycin is the treatment of choice. If first-line therapy fails, usually due to resistance, it can easily be followed with second-line or, occasionally, third-line chemotherapy, making the overall survival rate almost 100%.[6]
High-Risk Disease
Most high-risk GTN patients present with metastases months or years following the causative pregnancy. Signs and symptoms differ according to the disease's location. For example, patients with brain metastases may present with headaches, seizures, or hemiparesis. In contrast, patients with lung metastasis can present with shortness of breath, hemoptysis, or pleuritic chest pain. Since menstrual irregularity is not always present, the diagnosis can be missed. Recommended imaging studies are whole-body CT scans, MRI of the brain and pelvis, and Doppler ultrasonography. If the brain scan is negative, a lumbar puncture should be done to assess the ratio of cerebrospinal fluid to serum hCG.[6]
Complications
Complications of GTD can be surgical and/or medical. The surgical evacuation of a molar pregnancy should be performed as soon as the patient is medically stable.[38] A hospital with an intensive care unit, a blood bank, and anesthesia support is optimal for GTD patients with markedly enlarged uteri undergoing surgical evacuation.[44]
Commonly reported medical complications of a molar pregnancy include hyperemesis, hyperthyroidism, vaginal hemorrhage, anemia, preeclampsia, and respiratory distress.[6] Pulmonary complications occur less commonly yet are life-threatening. These complications include pulmonary edema, pulmonary embolism, pleural effusion, and trophoblastic embolization.[69]
In patients with a high hCG level or suspected hyperthyroidism, surgery and anesthesia can precipitate thyroid storm, and beta-adrenergic blockers should be administered.[70] Respiratory distress can occur during and/or after evacuation. Heavy bleeding with high fluid replacement may be a cause. Trophoblastic pulmonary embolization, preeclampsia, and thyroid storm with high-output heart failure can also lead to respiratory compromise. Anesthesia and recovery room staff must be aware of these potential complications.[71]
Without treatment, choriocarcinoma can result in death. With the advent of chemotherapy, many patients can achieve remission and cure of their disease. The utilization of chemotherapy is not without its risks, including the development of secondary malignancies, nausea, vomiting, hair loss, diarrhea, fevers, infections, and the need for transfusion of blood products.
Postoperative and Rehabilitation Care
Outpatient serum hCG levels are obtained weekly after surgery until an undetectable level of hCG is obtained. Effective contraception is strongly recommended in patients with a previous molar pregnancy, and oral contraceptives have been shown to be a safe option. A new pregnancy may interfere with weekly serum hCG level monitoring and make it impossible to determine the occurrence of invasive molar disease. Established guidelines for hCG monitoring should be closely followed.[45][72]
Deterrence and Patient Education
Healthcare providers are crucial in raising awareness about GTD and its risk factors. Educating patients about the importance of early prenatal care and regular follow-up visits can help detect GTD early, leading to better outcomes.
For patients diagnosed with GTD, providing clear and accurate information about the follow-up schedule and the importance of ongoing monitoring is essential. Educating patients about the need for regular hCG testing can help ensure the timely detection of any recurrent or persistent disease, leading to appropriate management and improved outcomes.[45][73]
Trophoblastic sequelae (invasive mole or choriocarcinoma) occur in nearly 15% to 20% of patients with complete HMs and 1% to 5% with partial HMs.[1] All patients with HMs should have hCG surveillance and monitoring. Surveillance protocols differ from one country to another, but the principles are identical. In the United Kingdom, serum and urine hCG levels are measured every 2 weeks until the values reach the negative range, then urine hCG levels are checked monthly. Patients who achieve negative hCG values within 56 days of uterine evacuation have a low risk of developing malignant disease and are further monitored monthly for 6 months from the evacuation date.
During the hCG follow-up period, patients are advised to use reliable contraception (ideally, a combination of methods). Following hCG monitoring, serum or urine hCG concentrations should be measured 6 and 10 weeks after every pregnancy to ensure no reactivation of previous molar disease.[6] Any woman who has given birth, particularly high-risk patients, should be counseled to return for continued post-partum bleeding. Future prenatal care should include early obstetric ultrasound.[39]
Pearls and Other Issues
Take-Home Points
As outlined below, partial and complete HMs have distinct clinical and pathological features.
Clinical Presentation
- Partial HM: Low presenting hCG level
- Uterus small for gestational age
- Rare theca lutein cysts
- Rare medical complications
- Often presents as a missed abortion in the absence of symptoms
- Ultrasound may demonstrate fetal parts
- Complete HM: High presenting hCG level
- Uterus 50% larger for gestational age
- Theca lutein cysts 15-25%
- Medical complications ≤25%
- Typically presents with heavy vaginal bleeding
- Classic ultrasound findings of a "snowstorm" or a "bunch of grapes"
Malignant Sequelae
- Partial HM: <5%
- Complete HM: Approximately 15% to 20%
Karyotype
- Partial HM: 69,XXX or 69,XXY
- Complete HM: 46,XX or 46,XY
Fetal Tissue
- Partial HM: Often present
- Complete HM: Absent
Villous Edema
- Partial HM: Variable, focal
- Complete HM: Diffuse
Trophoblastic Proliferation
- Partial HM: Focal
- Complete HM: Diffuse [5]
Elevated hCG levels in a woman without a confirmed intrauterine pregnancy should prompt a search for both ectopic pregnancy and the possibility of a malignancy secreting hCG. Lack of response to methotrexate makes a malignancy much more likely.[74]
False-positive elevations in hCG can be due to heterophile (human anti-mouse) antibodies and should be considered before treating patients surgically or with systemic chemotherapy.[1]
In men, choriocarcinoma usually occurs as part of a mixed germ cell tumor; when occurring in a pure form, the primary tumor may be very small or even regressed, while symptoms are all related to metastasis.[17]
Intra-placental choriocarcinoma is a very rare variant, usually presenting with metastatic symptoms in the postpartum mother and rarely with metastasis in the infant. It should be included in the differential diagnosis of elevated AFP in a postpartum patient.[75]
Enhancing Healthcare Team Outcomes
A molar pregnancy is best managed with an interprofessional team, including physicians, advanced practice practitioners, nurses, and pharmacists. Once a molar pregnancy is diagnosed and an obstetric/gynecology consultation is obtained, patients are typically admitted to the hospital for surgery. Patients with molar pregnancies must be monitored for associated complications, including hyperthyroidism, preeclampsia, and ovarian theca lutein cysts. Molar pregnancy-induced hyperthyroidism should resolve with the evacuation of the uterus, but patients may require beta-adrenergic blocking agents before anesthesia to reverse the effects of thyroid storm. Preeclampsia also resolves quickly after the evacuation of the uterus. Theca lutein cysts will regress spontaneously with falling hCG levels. However, patients must be counseled on the signs and symptoms of ovarian torsion and ruptured ovarian cysts. Special attention post-evacuation is necessary to monitor for fluid overload. For those with localized disease, the outcomes are good.[76][77]
Weekly serum hCG levels are obtained after surgery until an undetectable level of hCG is reached (usually performed in an outpatient setting). Patients with a previous molar pregnancy are advised to use reliable contraception to avoid a new pregnancy. It could conflict with weekly serum hCG level testing and make it impossible to determine the occurrence of invasive molar disease. The risk of invasive disease in a complete molar pregnancy is around 15% to 20%, and in a partial molar pregnancy is 1% to 5%.
Clinicians taking care of patients following molar pregnancy must remain aware and vigilant of the pitfall of heterophile (human anti-mouse) antibodies occurring in 3% to 4% of patients, resulting in falsely positive elevated hCG. The presence of heterophile antibodies can be teased out by serial dilution of serum, which will not show a parallel decrease with dilution or sending of serum and patient urine to a reference hCG laboratory.[1]
High clinical suspicion should be maintained for choriocarcinoma in women with hemoptysis and molar pregnancy, current or recent pregnancy, or irregular vaginal bleeding.[16] An interprofessional treatment center with nurses and clinicians specializing in gestational trophoblastic disease improves outcomes.
Management of GTN requires the collaboration of laboratory personnel, ultrasonographers, nurses, advanced practice practitioners, radiologists, pathologists, gynecologists, medical oncologists, and gynecologic oncologists. Healthcare professionals must work together to schedule and coordinate diagnostic tests, such as hCG measurements, imaging studies, and histopathological evaluations, to accurately diagnose GTN and determine disease staging. Subsequently, the interdisciplinary team develops individualized treatment plans based on the specific subtype and stage of GTN. In certain complex situations, referral to centers for trophoblastic disease may be necessary.
Regular communication and collaboration are essential to ensure ongoing assessment of patients' treatment responses. Adjustments to treatment plans are made based on multidisciplinary discussions and patients' individual needs, allowing for coordinated and shared decision-making. Comprehensive patient education on GTN and the critical hCG follow-up requirements are best addressed across medical disciplines. By setting interprofessional goals and standards, healthcare professionals can work together effectively to provide comprehensive, patient-centered care for individuals with GTN, optimizing treatment outcomes and improving the overall patient experience.[43][49]