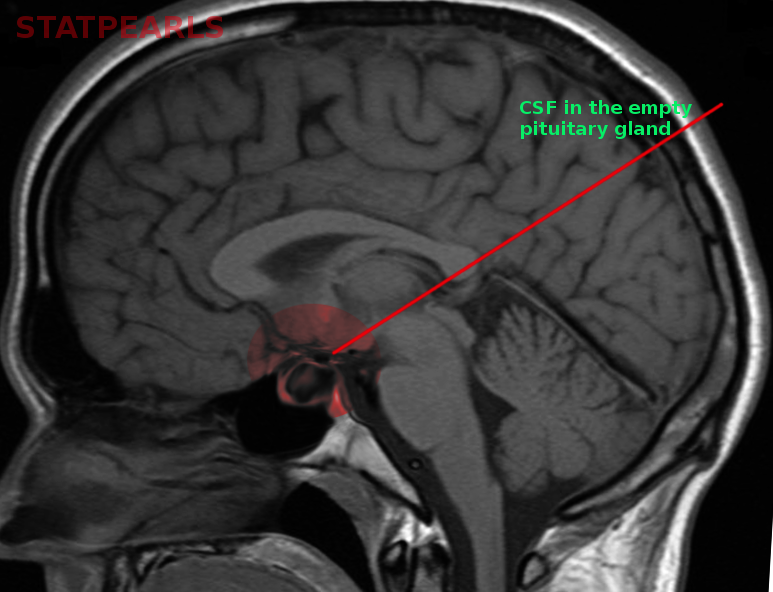
[1]
Auer MK, Stieg MR, Crispin A, Sievers C, Stalla GK, Kopczak A. Primary Empty Sella Syndrome and the Prevalence of Hormonal Dysregulation. Deutsches Arzteblatt international. 2018 Feb 16:115(7):99-105. doi: 10.3238/arztebl.2018.0099. Epub
[PubMed PMID: 29510819]
[2]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Miljic D, Pekic S, Popovic V. Empty Sella. Endotext. 2000:():
[PubMed PMID: 30321014]
[3]
Carosi G, Brunetti A, Mangone A, Baldelli R, Tresoldi A, Del Sindaco G, Lavezzi E, Sala E, Mungari R, Fatti LM, Galazzi E, Ferrante E, Indirli R, Biamonte E, Arosio M, Cozzi R, Lania A, Mazziotti G, Mantovani G. A Multicenter Cohort Study in Patients With Primary Empty Sella: Hormonal and Neuroradiological Features Over a Long Follow-Up. Frontiers in endocrinology. 2022:13():925378. doi: 10.3389/fendo.2022.925378. Epub 2022 Jun 23
[PubMed PMID: 35813618]
[4]
Chen T, Li G, Wu D, Xie B, Feng Y, Xiao S, Li J, Liu Y, Yang J, Li X. Primary empty sella: The risk factors and associations with the cerebral small vessel diseases-An observational study. Clinical neurology and neurosurgery. 2021 Apr:203():106586. doi: 10.1016/j.clineuro.2021.106586. Epub 2021 Mar 5
[PubMed PMID: 33730618]
Level 2 (mid-level) evidence
[5]
De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. The Journal of clinical endocrinology and metabolism. 2005 Sep:90(9):5471-7
[PubMed PMID: 15972577]
[6]
Chiloiro S, Giampietro A, Bianchi A, Tartaglione T, Capobianco A, Anile C, De Marinis L. DIAGNOSIS OF ENDOCRINE DISEASE: Primary empty sella: a comprehensive review. European journal of endocrinology. 2017 Dec:177(6):R275-R285. doi: 10.1530/EJE-17-0505. Epub 2017 Aug 5
[PubMed PMID: 28780516]
[7]
Guitelman M, Garcia Basavilbaso N, Vitale M, Chervin A, Katz D, Miragaya K, Herrera J, Cornalo D, Servidio M, Boero L, Manavela M, Danilowicz K, Alfieri A, Stalldecker G, Glerean M, Fainstein Day P, Ballarino C, Mallea Gil MS, Rogozinski A. Primary empty sella (PES): a review of 175 cases. Pituitary. 2013 Jun:16(2):270-4. doi: 10.1007/s11102-012-0416-6. Epub
[PubMed PMID: 22875743]
Level 3 (low-level) evidence
[8]
Saindane AM, Lim PP, Aiken A, Chen Z, Hudgins PA. Factors determining the clinical significance of an "empty" sella turcica. AJR. American journal of roentgenology. 2013 May:200(5):1125-31. doi: 10.2214/AJR.12.9013. Epub
[PubMed PMID: 23617499]
[9]
Zagardo MT, Cail WS, Kelman SE, Rothman MI. Reversible empty sella in idiopathic intracranial hypertension: an indicator of successful therapy? AJNR. American journal of neuroradiology. 1996 Nov-Dec:17(10):1953-6
[PubMed PMID: 8933886]
[10]
Akkus G, Sözütok S, Odabaş F, Onan B, Evran M, Karagun B, Sert M, Tetiker T. Pituitary Volume in Patients with Primary Empty Sella and Clinical Relevance to Pituitary Hormone Secretion: A Retrospective Single Center Study. Current medical imaging. 2021:17(8):1018-1024. doi: 10.2174/1573405617666210525111218. Epub
[PubMed PMID: 34036923]
Level 2 (mid-level) evidence
[11]
Schlosser RJ, Bolger WE. Spontaneous nasal cerebrospinal fluid leaks and empty sella syndrome: a clinical association. American journal of rhinology. 2003 Mar-Apr:17(2):91-6
[PubMed PMID: 12751703]
[12]
Takkavatakarn K, Wipattanakitcharoen A, Katavetin P, Eiam-Ong S. Severe hyponatremia as the presenting manifestation of primary empty sella syndrome. Clinical case reports. 2022 Feb:10(2):e05369. doi: 10.1002/ccr3.5369. Epub 2022 Feb 3
[PubMed PMID: 35140964]
Level 3 (low-level) evidence
[13]
Bestepe N, Aydin C, Tam AA, Ercan K, Ersoy R, Cakir B. EMPTY SELLA IN A PATIENT WITH CLINICAL AND BIOCHEMICAL DIAGNOSIS OF ACROMEGALY. Acta endocrinologica (Bucharest, Romania : 2005). 2022 Jan-Mar:18(1):97-101. doi: 10.4183/aeb.2022.97. Epub
[PubMed PMID: 35975262]
[14]
Giustina A, Aimaretti G, Bondanelli M, Buzi F, Cannavò S, Cirillo S, Colao A, De Marinis L, Ferone D, Gasperi M, Grottoli S, Porcelli T, Ghigo E, degli Uberti E. Primary empty sella: Why and when to investigate hypothalamic-pituitary function. Journal of endocrinological investigation. 2010 May:33(5):343-6
[PubMed PMID: 20208457]
[15]
Zuhur SS, Kuzu I, Ozturk FY, Uysal E, Altuntas Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: do all cases need endocrinological evaluation? Turkish neurosurgery. 2014:24(3):374-9. doi: 10.5137/1019-5149.JTN.8671-13.0. Epub
[PubMed PMID: 24848177]
Level 3 (low-level) evidence
[16]
Kuo M, Maya MM, Bonert V, Melmed S. Prospective Evaluation of Incidental Pituitary Imaging Findings in the Sella Turcica. Journal of the Endocrine Society. 2021 Feb 1:5(2):bvaa186. doi: 10.1210/jendso/bvaa186. Epub 2020 Nov 29
[PubMed PMID: 33392424]
[17]
Chen HC, Sung CC. A young man with secondary adrenal insufficiency due to empty sella syndrome. BMC nephrology. 2022 Feb 25:23(1):81. doi: 10.1186/s12882-022-02699-6. Epub 2022 Feb 25
[PubMed PMID: 35216554]
[18]
Garcia JM, Biller BMK, Korbonits M, Popovic V, Luger A, Strasburger CJ, Chanson P, Medic-Stojanoska M, Schopohl J, Zakrzewska A, Pekic S, Bolanowski M, Swerdloff R, Wang C, Blevins T, Marcelli M, Ammer N, Sachse R, Yuen KCJ. Macimorelin as a Diagnostic Test for Adult GH Deficiency. The Journal of clinical endocrinology and metabolism. 2018 Aug 1:103(8):3083-3093. doi: 10.1210/jc.2018-00665. Epub
[PubMed PMID: 29860473]
[19]
Alexandraki KI, Grossman A. Management of Hypopituitarism. Journal of clinical medicine. 2019 Dec 5:8(12):. doi: 10.3390/jcm8122153. Epub 2019 Dec 5
[PubMed PMID: 31817511]
[20]
Gadelha MR, Wildemberg LE, Lamback EB, Barbosa MA, Kasuki L, Ventura N. Approach to the Patient: Differential Diagnosis of Cystic Sellar Lesions. The Journal of clinical endocrinology and metabolism. 2022 May 17:107(6):1751-1758. doi: 10.1210/clinem/dgac033. Epub
[PubMed PMID: 35092687]
[21]
Mehta GU, Bakhtian KD, Oldfield EH. Effect of primary empty sella syndrome on pituitary surgery for Cushing's disease. Journal of neurosurgery. 2014 Sep:121(3):518-26. doi: 10.3171/2014.3.JNS132012. Epub 2014 May 23
[PubMed PMID: 24857241]
[22]
Winograd E, Kortz MW, Lillehei KO. Radiographic pituitary stalk disruption: A rare sequela of secondary empty sella syndrome. Surgical neurology international. 2021:12():385. doi: 10.25259/SNI_530_2021. Epub 2021 Aug 3
[PubMed PMID: 34513152]