Continuing Education Activity
Objectives:
Evaluate patients with diabetic foot infections.
Identify clinical features of diabetic foot infections.
Implement recommended management approaches for diabetic foot infections.
Apply interprofessional team strategies to improve care coordination and outcomes in patients with diabetic foot infections.
Introduction
Approximately 29 million people have diabetes in the United States, and approximately 25% of people older than 65 have diabetes. The incidence of diabetes worldwide is projected to increase by 55% over the next 20 years, so this problem is only going to get worse. Approximately 75% of diabetic neuropathies are distal symmetric polyneuropathy (DSP).[1] Once peripheral neuropathy develops, the annual incidence of ulcer formation increases from <1 % to >7%. The 3-year mortality for people with diabetes increases from 13% to 28% with an ulcer.
The other major factor in diabetic foot infections is compromised blood flow. In the presence of local trauma and microvascular disease, diabetic foot infections may vary from a simple case of cellulitis to full-blown gangrene.
Osteomyelitis occurs in 15% of ulcers, and 15% of those require amputation.[2] Approximately 60% of patients undergoing lower extremity amputation have diabetic foot ulcers as the underlying cause. Following a lower extremity amputation, the 5-year mortality jumps to 60%. Diabetic foot ulcers are precursors to amputation and mortality, and therefore, every effort should be made to prevent them.
Treating diabetic foot infections is difficult as the blood flow is compromised and the antibiotics usually cannot reach the diseased area. In many patients with diabetes, a foot infection will progress, and the time to resolution can be much more prolonged than for patients without diabetes.
Etiology
Multiple forces are at work in the development of diabetic foot ulcers.[3] The 4 main branches of pathophysiology include neuropathy, ischemia, nutritional dysfunction, and infection. A well-perfused foot is more resistant to ulceration and infection than one with peripheral vascular disease. In people with diabetes, this is further complicated by the diminished effectiveness of perfusion caused by autonomic neuropathy, where there is less recruitment of capillaries and shunting of blood around capillary beds.
Neuropathy not only causes a diminished sensation but a loss of sweat and oil glands that leads to dry, cracking skin and a diminished neuroinflammatory response to noxious stimuli.[1] Additionally, glycosylation of tendons leads to stiffening and shortening that can cause foot deformities (eg, claw toes and hammer toes) and Achille's tendon stiffening, which increases pressure on the forefoot.[4]
Appropriate footwear is also essential for all patients with diabetes mellitus. Trauma or pressure from ill-fitting shoes can quickly compromise blood flow and predispose the patient to infection. Diabetes also aggravates and potentiates any peripheral vascular disease.
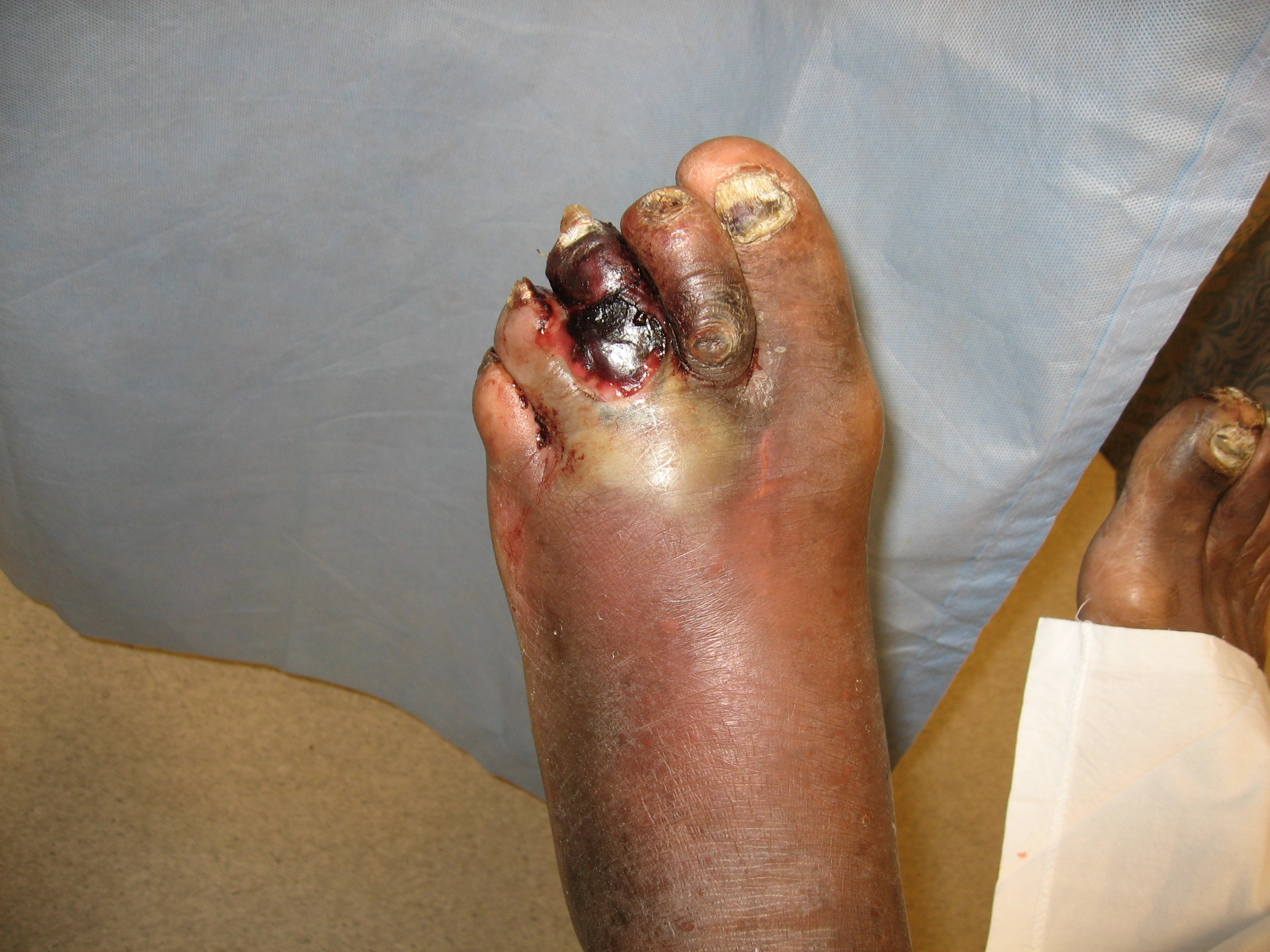
The organisms involved in a diabetic foot infection include staphylococcus, streptococci, pseudomonas, and many other anaerobes. In addition, gas-producing gram-negative organisms are also common in diabetic foot infections (see Image. Gas Gangrene of a Diabetic Foot).
Epidemiology
Diabetic foot infections are common infections in patients with diabetes. The incidence of diabetic foot infections is more common in elderly patients with comorbidities. Males and females are affected equally.
Mortality is rare except in unusual circumstances. The mortality risk is higher in patients with chronic osteomyelitis, those with acute necrotizing soft tissue infections, and those with additional underlying problems affecting the immune system.
Pathophysiology
Once a diabetic foot ulcer forms, it is a race against the clock to heal it before it gets infected. Like most skin and soft tissue infections, these start as Staphylococcal or Streptococcal infections, but as the depth and severity of the infection increases, they quickly become polymicrobial infections with gram-negative and anaerobic organisms.[5] People with diabetes have a diminished capacity to fight infection and have nutritional deficits that make healing a challenge.
History and Physical
When obtaining a clinical history from a person with diabetes, clinicians should screen for the risk factors of diabetic foot ulcers, including a history of an ulcer, sensory neuropathy, abnormal pulses, or peripheral vascular disease, foot deformity, age older than 65, poor glycemic control, end-organ damage, and renal disease. Patients known to be at risk for ulcers should be wearing offloading diabetic footwear to protect their feet at all times. Once an ulcer develops, knowing what the patient uses as footwear and any treatment strategies tried previously is essential.
The physical exam should include a thorough screening for peripheral vascular disease and sensory neuropathy and an evaluation of the depth and severity of the ulcer. All wounds should be thoroughly examined for evidence of chronic osteomyelitis. Penetrating ulcers may have deep sinus tracts. Finally, the pulses should be documented at each visit, and the ABI should be calculated.
Evaluation
Laboratory studies can help identify malnutrition (prealbumin), renal disease (BUN and creatinine), poor glycemic control (hemoglobin A1C), and screening for osteomyelitis (CRP >10 and ESR >40). When cultures are taken, obtaining a deep tissue culture rather than a superficial swab is essential because most wounds have superficial colonization, which is not necessarily representative of the most important infectious cause.[6]
Bone cultures are recommended to guide antibiotic selection in cases of osteomyelitis. Plain radiographs should be taken of all diabetic foot ulcers to screen for retained foreign bodies, osteomyelitis, and subcutaneous gas.
X-ray findings of osteomyelitis take 4 to 6 weeks to develop; therefore, if there is a high index of suspicion of osteomyelitis, magnetic resonance imaging (MRI) is helpful in making an early diagnosis. Computed tomography (CT) scans are useful for identifying deep space abscesses when an MRI is not readily available.
Transcutaneous oxygen measurements (TCOM) can be helpful in risk stratifying these patients. Transcutaneous oxygen tension of at least 40 mm Hg is needed for normal wound healing. Most patients with diabetes mellitus will have levels lower than this, and hyperbaric oxygen therapy and correction of peripheral vascular disease can increase oxygenation. Transcutaneous oxygen measurements can also predict who will most likely benefit from hyperbaric oxygen therapy and who needs critical reperfusion. A TCOM of >200 mm Hg under hyperbaric conditions indicates a greater likelihood of healing with a course of hyperbaric oxygen therapy with a sensitivity of 80% and a positive predictive value of 88%.[7]
Treatment / Management
Management strategies for wound care should include maintaining a moist wound environment, treating and preventing infection, offloading the affected area, debridement of necrotic tissue and biofilm, maximization of perfusion, nutrition, and oxygen delivery. Offloading strategies include debridement of calluses, padding, orthotics, therapeutic footwear, walking boots, total contact casts, and Achilles tendon lengthening.[8][9] Callous tissue increases the likelihood of ulcer formation 11-fold, and debriding the callous lowers the pressure exerted by 26%. The combination of Achilles tendon lengthening and total contact casts have the highest success of healing forefoot ulcers.[10][11]
Superficial wound infections can be treated with topical antimicrobials; however, systemic antibiotics will be required once cellulitis is present. Even mild peripheral vascular disease should be corrected in these patients to optimize their likelihood of healing. The duration of treatment with antibiotics usually varies from 2 to 4 weeks (see Image. Diabetic Foot Infection Treatment). Those with osteomyelitis may need at least 6 weeks of treatment.
Hyperbaric oxygen therapy can prevent amputations in patients with Wagner grade 3 or higher ulcers with a number needed to treat of 4.[12] Hyperbaric oxygen does this through various mechanisms, including increased oxygen delivery to ischemic/hypoxic tissues, enhanced white blood cell-mediated bacterial killing, angiogenesis, accelerated collagen synthesis and fibroblast growth, and decreased edema. Nine randomized controlled trials have shown the effectiveness of hyperbaric oxygen therapy in speeding healing, preventing amputations, and improving transcutaneous oxygen measurements. Hyperbaric oxygen increases the likelihood of wound healing with an odds ratio of 10. A standard treatment course is 30 to 40 treatments at 2.4 ATA, given once or twice daily.[12]
Surgical debridement is an essential treatment in many diabetic foot infections. The surgery involves debridement of the infected bone. In some cases, digital amputation may be required. A vascular surgeon should be consulted before undertaking any debridement because some patients may benefit from a bypass of the occluded vessel before debridement.
Osteomyelitis
Current guidelines regarding diabetic foot osteomyelitis management include:
- Control the soft tissue infection before resecting the bone. Drain all infected areas.
- Use negative pressure wound therapy dressings in between surgeries to help with healing.
- Resect the diseased bone until healthy bone is visible.
- Any infected area of bone seen on x-ray should be removed.
- All bone fragments removed at surgery should be examined for infection and organisms.
- Sample proximal bone to ensure it is infection-free.
- Use a power saw to resect bone.
- Delayed primary closure should take place once all infection is cured.
- Consider adjunctive tendo-Achilles lengthening in patients with ankle equinus deformity.
- If the patient has significant forefoot functional issues that may trigger ulcers, consult a podiatrist.
Differential Diagnosis
Differential diagnoses that should also be considered when evaluating diabetic foot infections include:
- Thrombophlebitis
- Phlegmasia cerulean dolens
- Compartment syndrome
- Acutely cold limb
- Venous stasis ulcer
Prognosis
The prognosis for a diabetic foot infection depends on many factors, including vascular blood supply and neuropathy. In many studies, 2 common risk factors for amputation include renal failure and significant peripheral vascular disease. Over the past 2 decades, the presence of MRSA has also been deemed to be a risk factor for the progression of the infection.
For patients who fail to control their blood glucose, the outcomes are poor, eventually resulting in an amputation. Even after a bypass, the cure is not guaranteed as the surgery is demanding and failures are common. The key reason for surgical bypass failures is that the target vessel is very small. Patients who do undergo a limb amputation are also at risk for adverse cardiac events and stroke. The overall quality of life of patients with diabetes mellitus who develop a foot infection is poor.
Complications
Complications of diabetic foot infections include:
- Osteomyelitis
- Bone fracture
- Sepsis
- Gangrene
- Necrosis
- Amputation
Pearls and Other Issues
Diabetic foot ulcers are precursors to amputations and require aggressive treatment. Patients should be screened for risk factors and referred for diabetic footwear when risk is identified. Staphylococcus aureus is the most critical pathogen in diabetic foot infections, but as the depth and severity increase, these become polymicrobial. Surgery is indicated for reversible peripheral vascular disease. Callouses should be debrided, and a moist wound healing environment should be maintained. Achille's tendon lengthening combined with total contact casting speeds healing and decreases the likelihood of recurrence, and hyperbaric oxygen therapy is indicated in selected cases (Wagner grade 3 or higher) to speed healing and prevent amputation.[13]