Continuing Education Activity
Dehydration is a condition caused by excessive loss of body water and, to varying degrees, electrolytes. Vomiting and diarrhea are the most common causes of dehydration in children. Treating children with dehydration remains a significant global challenge, particularly in resource-limited settings. Diarrheal diseases, which often lead to severe dehydration, are a leading cause of infant mortality worldwide, particularly among children aged under 5. This burden is disproportionately higher in developing countries, where patients may have limited access to healthcare resources and preventive measures.
This activity describes the etiology, pathophysiology, evaluation, prevention, and management of pediatric dehydration and highlights the importance of the interprofessional healthcare team collaborating to improve clinical outcomes. Collaboration among interprofessional health team members is crucial, especially in resource-limited countries. The WHO, in partnership with various agencies, promotes policies for safe drinking water, sanitation, and vaccination programs, while clinicians, community health workers, and pharmacists play vital roles in education, prevention, and treatment efforts.
Objectives:
Identify the signs and symptoms of dehydration and the underlying causes in children.
Differentiate between isonatremic, hyponatremic, and hypernatremic dehydration in children.
Implement treatment plans for children with dehydration based on hemodynamic status.
Collaborate with interprofessional team members to recognize, evaluate, treat, and monitor pediatric patients with dehydration.
Introduction
Globally, dehydration is a leading cause of pediatric morbidity and mortality. Diarrheal disease and dehydration cause 14% to 30% of deaths among infants and toddlers worldwide.[1] Viruses cause most cases of gastroenteritis in both developed and low-to-middle-income countries, and rotavirus is the most frequent etiology of gastroenteritis globally. In the United States, however, rotavirus hospitalizations have decreased since the licensing of the oral rotavirus vaccine in 2006, and now norovirus and other enteroviruses cause more gastroenteritis than rotavirus.
The World Health Organization (WHO) defines dehydration as a condition caused by excessive loss of body water. Infants and children have a higher percentage of total body water (TBW) than adults, approximately 65% to 80%. Young children are especially susceptible to dehydration because they cannot independently communicate their thirst to caregivers or access fluids. Infants have a relatively greater fluid requirement due to increased insensible fluid losses from their higher body surface area. Dehydration is classified into 3 categories depending on the value of the serum sodium: isotonic, hypotonic, and hypertonic. The etiologies and recommended treatments are different for each group, with the common goal of restoring water and electrolyte deficits, providing maintenance fluids, and replacing losses.
Etiology
Infants and young children are particularly susceptible to diarrheal disease and dehydration due to their higher metabolic rates, inability to communicate needs or hydrate themselves, and increased insensible losses.[2] Dehydration results from several disease processes that cause net fluid loss, including infective gastroenteritis, diabetic ketoacidosis (DKA), diabetes insipidus, burns, excessive sweating, and third spacing. In addition to TBW losses, electrolyte abnormalities frequently exist.
Most cases of dehydration result from acute infectious gastroenteritis. Viral infections, including rotavirus, norovirus, and enteroviruses, cause 75% to 90% of infectious diarrhea cases globally, and bacterial pathogens cause fewer than 20%. In children aged younger than 2, rotavirus is the most common causative organism. Since the licensing of the rotavirus vaccine in 2006, hospitalizations for dehydration have decreased, and the incidence of gastroenteritis caused by norovirus and other enteric viruses has increased. In resource-limited countries, some studies report a bacterial etiology of greater than 25% in children with gastroenteritis, most commonly enterotoxigenic Escherichia coli and Shigella.[3] Cryptosporidium, Aeromonas, Campylobacter jejuni, and Vibrio cholera are other frequently encountered pathogens.[4]
Clinicians sometimes blur the distinction between the terms dehydration and hypovolemia. Dehydration refers to a deficit of TBW, mainly from the intracellular compartment, leading to an imbalance in fluid and electrolyte levels. Dehydration occurs with inadequate fluid intake, excessive fluid loss through sweating, vomiting, diarrhea, or medical conditions such as DKA and diabetes insipidus. Dehydration causes thirst, dry mouth, dark urine, oliguria, dizziness, confusion, and tachycardia. Hypovolemia is a decrease in blood circulatory volume, specifically a reduction in blood plasma volume. This can occur due to hemorrhage, excessive sweating, severe burns, or fluid losses from the gastrointestinal tract. If not promptly treated, hypovolemia can cause hypotension, poor tissue perfusion, and shock. Both hypovolemia and dehydration involve fluid losses, but hypovolemia specifically refers to a reduction in blood volume, whereas dehydration is an overall deficit of TBW. These terms are sometimes interchanged in clinical practice as the conditions often coexist.
Epidemiology
Diarrhea and dehydration cause significant morbidity and mortality in infants and young children worldwide, annually causing approximately 700,000 to 800,000 pediatric deaths, representing nearly 16% of global child fatalities. Childhood diarrhea cases are concentrated in South Asia and Sub-Saharan Africa, and the WHO's diarrhea control program successfully reduced worldwide mortality by approximately 75% from the 1980s to 2008. Since then, progress in decreasing mortality has plateaued.[5] In Ethiopia, diarrheal disease kills an estimated 10,000 children aged under age 5 every year.[6] Inadequate sanitation, unsafe water, and lack of access to personal hygiene are responsible for most cases.[7] Pakistan has the highest infant mortality rate in Asia from diarrheal illnesses, with gastroenteritis and dehydration causing approximately 60% of child and infant deaths.[8] In the United States, diarrheal illnesses and dehydration account for more than 200,000 hospitalizations annually in young children but are not a leading cause of mortality. Globally, regions with high breastfeeding rates, safe water, adequate sanitation, and available rotavirus vaccines cause lower rates of pediatric diarrhea and dehydration requiring medical intervention.
Pathophysiology
Dehydration causes a decrease in TBW in both the intracellular and extracellular fluid volumes. Two-thirds of the TBW comprises the intracellular water and one-third the extracellular water. Extracellular water is distributed into the interstitial space (75%) and plasma (25%). TBW is higher in infants and children than adults, approximately 70% to 80% of total body weight in infants, 65% in children, and 45% to 60% in adults. Signs and symptoms of volume depletion, or decreased plasma volume, overlap with symptoms of dehydration. Dehydration refers to total water depletion, and volume depletion is the decrease in the circulatory volume, which occurs in acute blood loss, burns, sepsis, and anaphylaxis. Medical literature uses dehydration and volume depletion interchangeably, so this distinction remains important.[2]
Metabolic acidosis and dehydration frequently co-occur, and the pathophysiology is multifactorial. Acidosis can result from excess bicarbonate losses in diarrheal stools or urinary abnormalities resulting from conditions such as renal tubular acidosis and renal failure. Other disease processes causing metabolic acidosis include sepsis, DKA, ketosis of starvation, lactic acidosis due to inadequate tissue perfusion, and ingestion of toxins. Electrolyte abnormalities often accompany dehydration. For example, severe diarrhea with excessive potassium losses may cause hypokalemia, and infants with pyloric stenosis lose sodium and hydrochloric acid in the emesis, resulting in hypochloremic metabolic alkalosis.
History and Physical
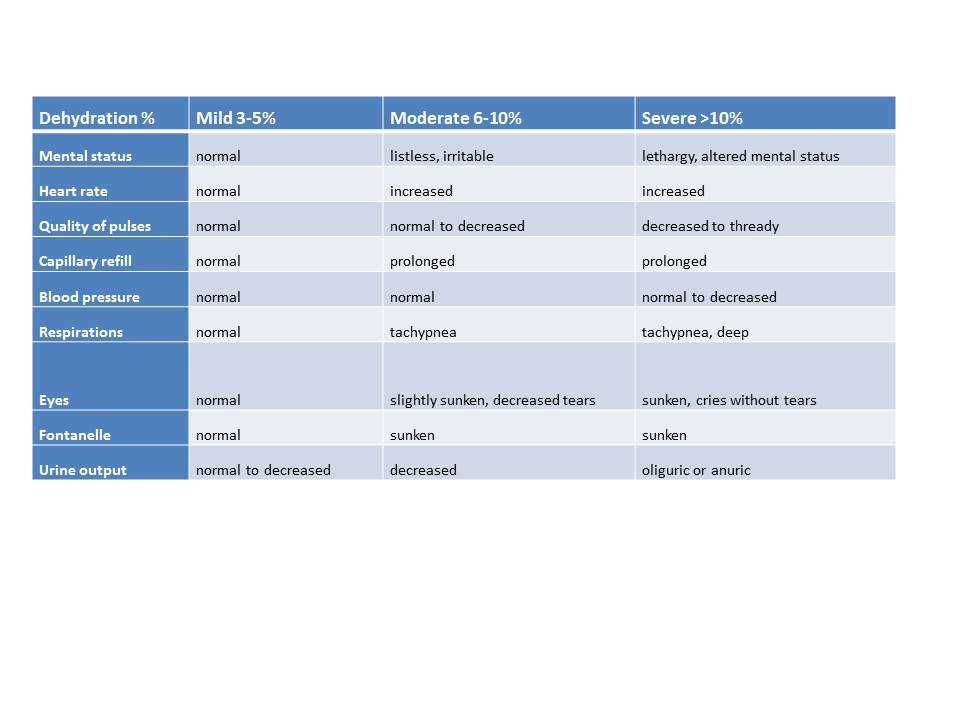
Signs and symptoms depend upon the patient's degree of dehydration, whether mild (3% to 5%), moderate (6% to 10%), or severe (more than 10%). The table below assists with categorizing the extent of dehydration. Because infants have a higher percentage of TBW than older children, 70% to 80% of body weight compared to 60% to 65%, they lose relatively more body weight at the same level of dehydration. Clinical assessment provides an approximation rather than an exact measurement, and knowing both the baseline and current weights is necessary to assess the degree of dehydration.
Patients with mild dehydration may only exhibit decreased urine output without other signs and symptoms. Dry oral mucosa, decreased skin turgor, prolonged capillary refill, tachycardia, and irritability occur with moderate dehydration. Children with severe dehydration appear extremely ill, with altered mental status and lethargy, hyperpnea, hypotension, and skin mottling, in addition to the previously mentioned signs. Hypotension and shock are late signs indicating poor organ perfusion in patients who require emergency fluid resuscitation. Patients with abdominal tenderness may have an acute abdominal process precipitating dehydration, requiring evaluation and treatment. Underlying illnesses contributing to dehydration must also be diagnosed and managed. Examples include jaundiced patients with hepatitis and ill children with gastrointestinal symptoms accompanied by the diffuse, macular rash of typhoid fever (see Image. Dehydration Scale By Percentage or Table below).[9]
Table. Dehydration Scale by Percentage
| Dehydration % |
Mild 3% to 5% |
Moderate 6% to 10% |
Severe >10% |
| Mental status |
Normal |
Listless, irritable |
Somnolent |
| Heart rate |
Normal |
Increased |
Increased |
| Pulses |
Normal |
Normal to decreased |
Decreased to thready |
| Capillary refill |
Normal |
Prolonged |
Prolonged |
| Blood pressure |
Normal |
Normal |
Normal to decreased |
| Respirations |
Normal |
Tachypnea |
Tachypnea |
| Eyes |
Normal |
Slightly sunken, decreased tears |
Few or no tears |
| Fontanelle |
Normal |
Depressed |
Sunken |
| Urine output |
Normal |
Decreased |
Oliguric or anuric |
Evaluation
Laboratory testing is indicated for patients requiring intravenous fluid administration. Children with mild dehydration usually have serum electrolytes and acid or base balance within normal limits. They may demonstrate concentrated urine with a specific gravity greater than 1.015 as the kidneys conserve water, but the presence of urine ketones usually suggests more severe dehydration. Elevated blood urea nitrogen (BUN) levels are also associated with worsening dehydration. However, other factors, such as excessive protein catabolism, high dietary protein, and gastrointestinal bleeding, can also contribute to elevations of BUN. The serum bicarbonate value is a relatively sensitive test indicating the degree of dehydration. A value of less than 17 mEq/L (17 mmol/L) is consistent with at least moderate dehydration.[1][10]
The measurement of serum sodium determines whether the child has isonatremic, hyponatremic, or hypernatremic dehydration and assists the clinician in choosing the type and rate of volume repletion. The serum sodium value reflects the relative loss of solute to TBW. In isonatremic dehydration, solute and TBW losses are proportionate, and the serum sodium value is 130 to 150 mEq/L (130 - 150 mmol/L). Hyponatremic dehydration occurs when water or hypotonic fluids replace losses from diarrhea, and the serum sodium is less than 130 mEq/L (130 mmol/L). Severe hyponatremia develops in the syndrome of inappropriate secretion of antidiuretic hormone (SIADH), causing seizures. In hypernatremic dehydration, TBW loss surpasses solute loss, and the serum sodium exceeds 150 mEq/L (150 mmol/L). Hypernatremia occurs with severe viral gastroenteritis, especially in infants unable to replace ongoing losses or those ingesting oral rehydration solutions prepared with insufficient water. Medical conditions that impair the ability to regulate water balance, such as diabetes insipidus and renal disorders, can also cause hypernatremic dehydration.
Serum potassium levels may be abnormal in patients with dehydration, most frequently hypokalemia due to diarrheal stool losses. With significant bicarbonate losses from stool, metabolic acidosis develops and can progress to poor tissue perfusion and ketosis. When hypovolemia and metabolic acidosis co-occur, the serum potassium rises as potassium shifts from the intracellular to the extracellular space. Monitoring serum potassium levels is essential to guide therapy and avoid complications of hyperkalemia, such as cardiac dysrhythmias.
Children with severe vomiting can develop metabolic alkalosis caused by loss of hydrochloric acid from the stomach, typically in infants with untreated pyloric stenosis. Laboratory results of patients with vomiting and dehydration may also show hypoglycemia in addition to electrolyte abnormalities. Ongoing trials are studying end-tidal carbon dioxide measurements to assess the severity of dehydration. Although this non-invasive approach has promise, no proven tool determines the degree of dehydration in pediatric patients.[11]
Treatment / Management
Priorities in managing dehydration include the early recognition of symptoms, identifying the degree of dehydration, restoring water and electrolyte deficits, replacing ongoing losses, and maintaining fluids. Measuring the volume of emesis and stool estimates continuing deficits that help determine the route, volume, and rehydration rate. Two treatment phases include replacing the deficit of fluid and electrolytes, which continues until signs and symptoms of dehydration improve and the patient urinates. The maintenance phase follows the replacement phase, usually about 4 hours later, when additional fluids provide basal metabolic needs and restore ongoing losses.[9][10][12]
Mild to Moderate Dehydration
The WHO and The American Academy of Pediatrics (AAP) recommend oral rehydration for mild to moderate dehydration, and the goal is to return patients to a euvolemic state. In the clinical setting, distinguishing between mild and moderate dehydration may occur because the symptoms overlap, and premorbid weights are often unknown. Breastfeeding infants and children should continue to nurse, and clinicians should first consider treatment with oral rehydration for older children with mild to moderate dehydration. [WHO. Pediatric Emergency Assessment]
Oral rehydration therapy (ORT) costs less than parenteral fluid administration, requires fewer technical skills for intravenous access, avoids complications like phlebitis and infection, and allows non-medical caregivers to participate in the treatment plan. ORT must be given in small amounts, frequently, to prevent triggering vomiting. Five milliliters every 1 to 2 minutes is usually well tolerated, but this approach is labor-intensive and requires a caregiver at the bedside. A 50 to 100 mL/kg volume is administered over 3 to 4 hours for mild-to-moderate dehydration. Nurses can insert a nasogastric tube for patients who refuse fluids or cannot drink. Children younger than 2 with mild dehydration should receive an additional 50 to 100 mL of fluid for each episode of vomiting or diarrhea. Age, weight, and estimated or measured losses guide the replacement volume in older children. Generally, clinicians should replace 10 to 20 mL/kg body weight for each diarrheal stool. As vomiting resolves, oral intake of small amounts of solids can begin, advancing slowly to a regular diet based on age. Patients who improve with ORT receive fluids and calories during maintenance, gradually returning to an unrestricted diet. During this phase, staff must frequently reassess hydration status and replace ongoing losses hourly.
The WHO recommends ORT solutions with a total osmolarity of 200 to 310 mOsm/L, glucose content of less than 20 g/L, sodium content of 60 to 90 mEq/L, and potassium content of 15 to 25 mEq/L. This balanced mixture of electrolyte salts and glucose is available as a pharmaceutical product distributed in packets. In many countries, other oral rehydration solutions (ORS) with similar concentrations are available in powdered, liquid, or "ice-pop" forms, often sweetened with sucralose for increased palatability. Fluids with high sugar content, including juice, soda, sweet tea, and sports drinks, might worsen diarrhea due to the unabsorbable glucose load and decreased water absorption by the intestinal lumen. Broths with excessive salt can cause hypernatremia. The WHO and AAP recommend standard, prepared ORS to avoid complications from drinking homemade preparations and everyday beverages.[13] ORT is contraindicated in patients with altered mental status and at risk for aspiration or those with abdominal ileus, malabsorption, persistent vomiting, or severe dehydration requiring higher fluid volumes.
Severe Dehydration
Clinicians must assess severely dehydrated patients and answer the following questions to determine the best treatment strategy: Is emergency management required? Has a trial of ORT failed, or is the child too dehydrated to attempt enteral hydration? If intravenous fluids are appropriate, what type of fluid should be selected? What initial volume should be administered for rehydration, and how will ongoing losses be calculated? What is the recommended infusion rate?
Severe dehydration with hypovolemia requires rapid volume repletion, beginning with isotonic saline (0.9% normal saline) 20 mL/kg, delivered within 20 minutes as an intravenous bolus and repeated based on clinical assessment. Children in hypovolemic shock may need multiple boluses. The WHO recommends fluid volumes of 70 to 100 mL/kg over 3 to 6 hours.[14] If intravenous access is unavailable, an intraosseus saline bolus can be administered as an emergency measure. Additional management includes obtaining a point-of-care glucose test, serum electrolytes, and urinalysis to assess specific gravity and the presence of ketones.[15] Venous blood gas, urea nitrogen, and serum creatinine measurements provide helpful information to guide further therapy.
Intravenous glucose, 0.5 to 1 g/kg, can correct hypoglycemia. A practical guideline is administering 5 to 10 mL/kg of a 10% dextrose solution (D10) or 2 to 4 mL/kg of a 25% dextrose solution (D25). Adolescents with a large bore intravenous line in place can receive 1 to 2 mL/kg of a 50% dextrose solution (D50).[16] 0.9% saline is the preferred initial resuscitation fluid for volume replacement. Alternatives include balanced isotonic solutions such as lactated Ringer solution, which is not clinically superior to normal saline.[17] Dextrose is generally not recommended for the replacement phase of fluid administration unless documented hypoglycemia exists.
The second, or maintenance phase, begins when the fluid and electrolyte deficits have improved or resolved, usually within about 4 hours. This phase lasts about 24 hours when the type of fluid administered depends on the patient's serum sodium concentration. Most cases of dehydration caused by gastroenteritis are isonatremic, and the serum sodium is between 130 and 150 mEq/L. Hyponatremia is a serum sodium less than 130 mEq/L; hypernatremia is a sodium greater than 150 mEq/L. Half of any remaining fluid deficit is administered for the first 8 hours of this second phase, with one-third of the daily maintenance requirement and additional fluid as needed to account for ongoing losses. During the next 16 hours, the remaining (if any) deficit and two-thirds of the daily maintenance fluids are given.
The overall fluid deficit is calculated by subtracting the patient's "sick weight" from their previous "well weight." One kilogram of weight loss is equivalent to 1 liter of fluid that must be replaced. Unfortunately, the premorbid is often unknown, and clinicians must estimate the deficit from signs, symptoms, and laboratory data. The WHO guidelines recommend that for children with severe (greater than 10%) dehydration, the estimated fluid deficit is 100 mL/kg or greater, and for those with moderate dehydration (5% to 10%), 50 to 100 mL/kg. The Holliday-Segar method calculates maintenance fluid needs in children, which are 100 mL/kg/day for the first 10 kg body weight, then 50 mL/kg/day for the next 10 kg, followed by an additional 20 mL/kg/day for additional body weight above 20 kg. Clinicians can calculate fluids needed to replace ongoing losses from vomiting and diarrhea, as described above in the paragraph on mild to moderate dehydration.[18]
In children with isonatremic dehydration requiring intravenous fluids, isotonic saline provides replacement water and sodium in appropriate proportions. Consuming hypotonic beverages, such as water, tea, or juice, can cause hyponatremia, and correcting the fluid volume deficit with normal saline raises the serum sodium concentration simultaneously. Hypokalemia may accompany hyponatremic dehydration, and adding potassium, 20 to 40 mEq/L of normal saline, replenishes intracellular potassium and facilitates the movement of sodium into the extracellular fluid, correcting the serum sodium level. Clinicians should verify that patients urinate before adding potassium to intravenous fluids.
Symptomatic hyponatremia (seizures, lethargy) can be acutely managed with hypertonic saline (3% sodium chloride). The deficit to restore the serum sodium to 130 mEq/L, administered over 48 hours, can be calculated by the following equation, where SNa = serum sodium concentration:
Sodium deficit = (130 - SNa) x volume of distribution x weight in kg
Example: If the SNa = 123, weight = 10 kg, and the volume of distribution is 0.6, the sodium deficit = (130-123) x 0.6 x 10 kg = 42 mEq sodium. Hypertonic saline (3%) containing 0.5 mEq/mL of sodium can partially correct symptomatic hyponatremia. A 4 mL/kg bolus dose raises the serum sodium by 3 to 4 mEq/L.
When hypernatremic dehydration occurs, deficits of water and sodium exist with higher losses of free water due to various causes, such as high fever, sweating, inability to concentrate the urine, or watery diarrhea. The serum sodium level rises because relatively more water is lost than sodium. The free water deficit is the additional water loss sufficient to elevate the serum sodium level above normal limits. The total water deficit is the sum of the free and isotonic, or solute, fluid deficits. Knowing the approximate free water deficit guides administering fluids after the initial volume resuscitation with isotonic saline, including some hypotonic fluids.[19]
The following 2 formulas estimate the FWD. These are approximations, and treatment relies on frequent clinical assessment and monitoring of laboratory data.
1. FWD = TBW [(SNa/140) -1]. For example, if a 10 kg child is 10% dehydrated with a serum sodium concentration of 156 mEq/L, the premorbid TBW is 0.6 x 10 kg = 6 L, and the current weight is 9 kg. The total fluid deficit is 1 L (10% of 10 kg). FWD = 6L [(156/140 mEq/L) -1] = 686 mL.
2. FWD = 4 mL/kg x weight in kg x (SNa-140). For the same example, the calculation is as follows: 4mL/kg x 10kg x (156-140) = 640 mL.
Therefore, the TWD is 1000 mL, comprised of 686 mL free water deficit and 314 mL of isotonic deficit using the first formula, and 1000 mL, 640 mL, and 360 mL using the second formula. If a 20 mL/kg bolus of 200 ml isotonic saline was administered initially, then the remaining isotonic deficit is only 114 mL (or 160 mL with the second formula). Rapid correction of hypernatremia can cause neurologic sequelae, including seizures. Normalizing the serum sodium by less than or equal to 0.5 mEq/hour over 36 to 48 hours is generally a safe rate to avoid adverse neurologic effects.[20]
Nasogastric hydration is an alternative for children incapable of taking fluids orally. Subcutaneous fluid administration is another method used when intravenous access cannot be achieved or maintained. Hypodermoclysis, the infusion of fluids and electrolytes via subcutaneous tissue, is employed mainly in older patients with chronic conditions but is not contraindicated in children. In low-resource settings, the advantages include low cost and ease of administration, minimal equipment and technical support needed, reduced discomfort from multiple attempts to insert an intravenous line, and a low incidence of infection. The procedure entails the placement of a topical anesthetic cream before inserting a 24- or 25-gauge catheter subcutaneously, usually between the scapulae. If available, hyaluronidase, 150 units, can be injected subcutaneously before the isotonic fluids to increase tissue permeability, facilitate the distribution of fluids, and reduce local swelling. Then, 20 mL/kg of fluid is administered, usually over 1 hour, until patients tolerate oral fluids or intravenous access is obtained.[21]
Differential Diagnosis
Clinicians must differentiate the multiple causes of dehydration in children before arriving at a final diagnosis. Dehydration commonly results from fluid loss due to vomiting, diarrhea, or inadequate oral intake but can also indicate underlying medical conditions that must be diagnosed and treated. These include infectious causes such as viral or bacterial gastroenteritis, urinary tract infections, or sepsis or shock, which exacerbate fluid loss and cause electrolyte imbalances.
Metabolic disorders, such as diabetes insipidus, ketoacidosis from diabetes mellitus, and congenital adrenal hyperplasia, with impaired water and electrolyte regulation, may also cause dehydration. Structural abnormalities, including pyloric stenosis or malrotation with volvulus, may obstruct gastrointestinal function, leading to fluid loss and subsequent dehydration. A comprehensive clinical assessment, including a history, physical examination, and appropriate laboratory investigations, is essential to diagnose and effectively manage pediatric dehydration.
Prognosis
Though dehydration remains a significant cause of pediatric morbidity and mortality globally, especially in resource-limited settings where access to clean water and healthcare infrastructure is limited, the prognosis depends on several factors. Prompt recognition and appropriate rehydration management, including ORS and intravenous fluids, can significantly improve outcomes. However, in regions with inadequate healthcare resources, severe dehydration poses considerable risks, leading to complications such as electrolyte imbalances, organ failure, and death. Recurrent episodes of dehydration, often associated with repeated gastrointestinal infections, can contribute to long-term poor health and stunted growth in children. Addressing underlying factors, including access to clean water, sanitation, immunizations, and the availability of preventive healthcare, remains crucial for reducing the global burden of pediatric dehydration and the associated morbidity and mortality.
Complications
Understanding the potential complications of pediatric dehydration is essential for clinicians caring for children. Dehydration can result in life-threatening complications. Electrolyte imbalances, such as hyponatremia or hypernatremia, can cause neurological sequelae, including confusion, seizures, and coma. Acute kidney injury may result from decreased renal perfusion, leading to anuria and the retention of metabolic waste products. Severe dehydration can cause life-threatening hypovolemic shock, characterized by hypotension and impaired tissue perfusion. Prolonged dehydration with delayed intervention can lead to metabolic acidosis, cardiac arrhythmias, and death. Recurrent, severe episodes of diarrhea are associated with malnutrition, resulting in cognitive and physical developmental delays and higher morbidity and mortality rates from dehydration.
Treatment of dehydration may also lead to complications. Improperly prepared ORS can cause electrolyte imbalances such as hypernatremia.[22] Rarely, gastrointestinal disturbances, including hemorrhage from gastric and duodenal ulcers following the consumption of incorrectly formulated ORS, have been reported.[23] Intravenous rehydration therapy requires frequent serum glucose and electrolyte monitoring to prevent or correct imbalances and guide ongoing clinical assessment. Correcting hypernatremia too quickly may cause cerebral edema and seizures.
Osmotic demyelination syndrome, also known as central pontine myelinolysis, can occur as a result of rapid correction of hyponatremia. Symptoms include headache, confusion, altered consciousness, and gait disturbance, and may lead to respiratory arrest. Other complications of delivering parenteral fluids too quickly are congestive heart failure and pulmonary edema, and intravenous therapy, in general, is accompanied by risks of phlebitis, infiltration, hematomas, cellulitis, and catheter-associated sepsis.[24] Clinicians must anticipate and promptly recognize the signs and symptoms of dehydration and initiate appropriate oral or parenteral rehydration therapy with close monitoring to prevent these serious complications.
Deterrence and Patient Education
Preventing pediatric dehydration is crucial for improved global health, especially in low-resource settings. Key preventive measures include immunizations against rotavirus, measles, and cholera, which significantly reduce the incidence of diarrheal illnesses, leading causes of dehydration in children. Exclusive breastfeeding for the first 6 months of life, followed by continued breastfeeding for up to 2 years, as the World Health Organization (WHO) recommends, provides essential nutrients and immunological protection. Ensuring access to clean water, improved hygiene, and proper sanitation, including using latrines and promoting handwashing, are cost-effective strategies that significantly reduce the risk of dehydration from diarrheal diseases. Despite the proven effectiveness of ORT, barriers persist, such as a lack of knowledge and compliance among clinicians who do not adhere to guidelines from the AAP, WHO, and CDC. Additionally, the expense of commercially prepared ORT may be unfeasible for home use in many regions. Addressing these barriers through education, training, and affordable ORT solutions is essential for improving pediatric health outcomes globally.
Pearls and Other Issues
Clinical pearls that assist in providing comprehensive and effective care for pediatric patients with dehydration, particularly in resource-limited settings, include the following:
- Patients with known or suspected cholera or shigella causing dehydration should be treated with appropriate antibiotics, which can reduce the duration and severity of the illness and prevent complications.
- Obtaining stool cultures can identify bacterial etiologies of infectious gastroenteritis, enabling targeted antibiotic therapy and infection control.
- Stool examination for ova and parasites may identify pathogens like Entamoeba histolytica, treatable with metronidazole. This is particularly relevant in regions where parasitic infections are common.
- In areas with a high prevalence of zinc deficiency, zinc supplements may benefit children aged older than 6 months with diarrhea and dehydration. Zinc may reduce the severity and duration of diarrheal episodes.
- Anti-emetics may facilitate ORS use in children with severe vomiting and prevent the need for parenteral fluid replacement. Ondansetron is commonly used to improve the success of oral fluid therapy.
- To maintain nutritional status and promote recovery, continue breastfeeding and provide age-appropriate food during rehydration. Malnutrition exacerbates dehydration and delays recovery.
Enhancing Healthcare Team Outcomes
Collaboration among interprofessional health team members is crucial in the global fight against pediatric dehydration and diarrheal diseases, which are the leading causes of infant mortality worldwide, particularly in children aged under 5. This burden is especially severe in resource-limited countries and requires comprehensive cooperation between various agencies and governments to reduce morbidity and mortality. In collaboration with member countries and other organizations, the WHO leads efforts to advocate for national policies and investments ensuring access to clean drinking water and improved sanitation. The organization also supports research on preventing diarrhea and implementing vaccination programs.
Government agencies, non-governmental organizations (NGOs), and international bodies like the United Nations International Children's Emergency Fund (UNICEF) play pivotal roles in these initiatives. They support implementing preventive measures, including water treatment, safe food storage practices, community hygiene education, and vaccine distribution. Partnerships with private sector organizations leverage additional expertise, resources, and innovation in water purification, nutrition, and pharmaceuticals. Clinicians, including nurses and lactation consultants, are vital in promoting exclusive breastfeeding for the first 6 months of life and continued breastfeeding for up to 2 years, boosting immunity and reducing the risk of dehydration. Regular meetings involving all team members, including clinicians, non-governmental organizations, government agencies, and community leaders, can help align goals, share updates, identify areas for improvement, and address challenges collaboratively. They can advocate for policies that support interprofessional collaboration, adequate funding, and sustainable health interventions at local, national, and international levels.
Nurses and community health workers are often on the front lines, educating parents about the importance of hygiene, the clinical signs of dehydration, and administering oral rehydration therapy (ORT). Community health workers and non-medical personnel can perform specific tasks, such as distributing ORS and clean water and extending the reach of healthcare services in resource-limited settings. Pharmacists supply and manage intravenous fluids and anti-emetic medications, ensuring critical resources are available and used appropriately. Water specialists evaluate and provide safe drinking water, while immunization programs supported by organizations like UNICEF prevent rotavirus, cholera, and other vaccine-preventable diarrheal diseases.
Parents and caregivers also play a crucial role in preventing dehydration by practicing hygiene practices at home, such as proper handwashing, safe food preparation, and recognizing early symptoms of dehydration. Implementing these measures and educating communities is fundamental to achieving sustainable improvements. This global interprofessional team effort, integrating diverse expertise and resources, is critical to effectively preventing and treating a preventable medical condition.