Continuing Education Activity
Coronary artery disease (CAD) is a prevalent heart condition characterized by the buildup of atherosclerotic plaque within the arterial lumen. Blood flow impairment reduces oxygen delivery to the myocardium. CAD is the most common cause of major morbidity and mortality in the US and worldwide. Prompt diagnosis and treatment improve outcomes.
The evaluation of CAD typically involves a combination of clinical assessment and diagnostic tests such as electrocardiography, stress testing, and echocardiography. Coronary angiography helps assess the extent of coronary blockages.
Treatment options for CAD range from lifestyle changes and medications to more invasive procedures. Medications like statins, antiplatelet agents, and β-blockers aim to manage symptoms and reduce further plaque buildup. In severe cases, interventions such as percutaneous coronary intervention and the more invasive coronary artery bypass grafting may be necessary. Complications of untreated or poorly managed CAD include heart failure, arrhythmias, and sudden cardiac death.
This activity for healthcare professionals is designed to enhance learners' proficiency in evaluating and managing CAD. Participants gain a deeper insight into the condition's risk factors, pathogenesis, and evidence-based diagnostic and therapeutic strategies. Greater competence enables clinicians to collaborate within an interprofessional team caring for patients with CAD.
Objectives:
Identify the etiology of coronary artery disease.
Create a clinically guided strategy for evaluating coronary artery disease.
Implement individualized therapeutic plans for patients with coronary artery disease.
Collaborate with the interprofessional team to educate, treat, and monitor individuals with coronary artery disease to improve health outcomes.
Introduction
Coronary artery disease (CAD) is characterized by the development of atherosclerosis in the coronary arteries, which can sometimes be asymptomatic. Coronary heart disease (CHD), also known as ischemic heart disease (IHD), encompasses conditions such as stable angina, acute coronary syndrome (ACS), and silent myocardial ischemia. Mortality associated with CHD is mainly due to CAD. ACS is typically symptomatic and includes conditions like unstable angina and myocardial infarction. For clarity, we will refer to CHD as "CAD" throughout this discussion.
CAD is the leading single cause of death and disability-adjusted life years (DALYs) lost worldwide. This burden disproportionately affects low- and middle-income countries, contributing to approximately 7 million deaths and 129 million DALYs annually. In 2015, CAD was responsible for 8.9 million deaths and 164.0 million DALYs globally. Individuals who survive myocardial infarction face a significantly higher risk of recurrent events and have an annual mortality rate 5 to 6 times higher than people without CAD.[1]
CAD is marked by an inadequate supply of blood and oxygen to the myocardium. The condition arises from occlusion of the coronary arteries and results in a demand-supply mismatch of oxygen. CAD typically involves the formation of plaques in the lumen of coronary arteries that impede blood flow.
CAD is the major cause of death in the US and worldwide. CAD was an uncommon cause of death at the beginning of the 20th century. Deaths due to CAD peaked in the mid-1960s and then decreased. However, This disease is still the leading cause of death worldwide.[2] The likelihood of death from CAD increases substantially with age. Among the modifiable risk factors, systolic blood pressure plays a crucial role, accounting for a portion of the heightened CAD risk associated with aging.[3]
Etiology
CAD is multifactorial. Etiologic factors can be broadly categorized into nonmodifiable and modifiable. Nonmodifiable factors include gender, age, family history, and genetics. Modifiable risk factors include hypertension, smoking, obesity, lipid levels, and psychosocial variables.[4] In the Western world, a faster-paced lifestyle has led people to eat more fast foods and unhealthy meals, which has paved the way for the increased prevalence of IHD. In the US, better primary care in the middle and higher socioeconomic groups has pushed the incidence toward the later part of life. Smoking remains the number one cause of cardiovascular disease. In 2016, the prevalence of smoking in the United States among adults was found to be 15.5%.[5]
Men are more predisposed to CAD than women. Hypercholesterolemia remains an important modifiable risk factor for CAD. Increased low-density lipoproteins (LDL) raise CAD risk, and elevated high-density lipoproteins (HDL) decrease CAD incidence. An individual's 10-year risk of atherosclerotic cardiovascular disease can be calculated using the atherosclerotic cardiovascular disease equation on the American Heart Association (AHA) portal.[6][7] Markers of inflammation are also substantial risk factors for CAD. High-sensitivity C-reactive protein (hs-CRP) is considered the best predictor of CAD in some studies, although its practical uses are controversial.[8]
Research indicates that obesity is linked to increased cardiovascular disease risk and a higher prevalence of cardiovascular conditions.[9][10] Waist circumference indicates abdominal fat accumulation better than body mass index (BMI). Individuals with a normal BMI but increased waist circumference are at greater risk for developing CAD. The current obesity guidelines do not provide specific recommendations for waist circumference cut-off values in patients with CAD and a normal BMI. Sharma et al examined data from 7,057 patients with CAD across 5 cohort studies. The group found that a higher waist circumference or waist-to-hip ratio, despite having a normal BMI, was associated with an increased risk of mortality in patients with CAD.[11]
Epidemiology
Although the incidence of CAD has been declining in developed nations, the overall number of coronary events is not expected to decrease due to factors like immigration and aging. These factors may lead to a transient increase in CAD prevalence. In contrast, developing countries show significant variability in CAD incidence. The spread of Western dietary habits and a more sedentary lifestyle will likely contribute to a sharp rise in CAD cases in these regions.
The continued reduction in CAD mortality rates in developed countries over recent decades can be attributed to advancements in acute treatment and the success of both primary and secondary preventive strategies. However, outcomes may still vary based on ethnic differences, social inequalities, and disparities in access to effective treatments and preventive measures within different regions of the same country. This variation underscores the need for further research and evaluation across various areas.[12]
One study estimated that CAD represented 2.2% of the global disease burden and 32.7% of cardiovascular diseases. The condition costs over 200 billion dollars annually to the health care system in the United States. An estimated 7.6% of men and 5.0% of women in the US lived with CAD from 2009 to 2012, based on the national health survey conducted by the AHA. Thus, about 15.5 million Americans are afflicted with the disease during this time.[13][4] In 2020, an estimated 19.05 million deaths worldwide were attributed to CVD, indicating an 18.71% rise since 2010. The age-standardized death rate per 100,000 people was 239.80, reflecting a 12.19% decrease over the same period. The overall crude prevalence of CVD reached 607.64 million cases in 2020, representing a 29.01% increase compared to 2010.[14]
The incidence of CAD rises with age, regardless of gender. In France's ONACI registry, the incidence of CAD was about 1% in individuals aged 45 to 65, increasing to about 4% in people aged 75 to 84 years.[15]
Multiple studies have demonstrated that the presence of cerebral (CeVD) and peripheral vascular disease (PVD) significantly increases the risk, morbidity, and mortality associated with CAD. For instance, the Gulf Registry of Acute Coronary Events, which included 7,689 patients with ACS, highlighted that polyvascular disease is linked to higher in-hospital and 1-year mortality rates, as well as a decrease in the prescription of dual antiplatelet therapy (DAPT).[16]
Similarly, the MASCARA registry, a multicenter study conducted across 32 hospitals in Spain, found that CeVD and PVD independently predicted increased in-hospital and 6-month mortality following ACS.[17] Ferreira-González et al also observed more severe CAD in patients with CeVD or PVD.[18] These findings are supported by additional studies, including one in Sweden involving 544 postmyocardial infarction patients, where individuals with severe CAD and polyvascular disease exhibited particularly high rates of cardiovascular death and hospital readmission.[19][20]
Pathophysiology
The hallmark of the pathophysiology of CAD is atherosclerotic plaque formation. Plaque is a buildup of fatty material that narrows the arterial lumen and impedes blood flow. The first step in the process is the formation of a "fatty streak" by subendothelial deposition of lipid-laden macrophages, also called "foam cells." When a vascular insult occurs, the intima layer breaks, and monocytes migrate into the subendothelial space, where they become macrophages. These macrophages take up oxidized LDL particles, leading to foam cell formation. T cells get activated, and cytokines are released to aid in the inflammatory process. Growth factors activate smooth muscles, which also take up oxidized LDL particles and collagen, deposit along with activated macrophages, and increase the population of foam cells. Subendothelial plaque subsequently develops.
The plaque could grow or become stable over time if no further endothelial insult occurs. A fibrous cap forms if the plaque becomes stable and the lesion calcifies over time. The lesion can become hemodynamically significant as time passes. Myocardial tissue perfusion can become insufficient, triggering angina symptoms during times of increased demand (eg, exercise) if the lumen has at least 70% obstruction. Symptoms often abate at rest as the oxygen requirement falls. Angina may occur at rest if the coronary artery is 90% stenosed. Some plaques can rupture and expose tissue factor, culminating in thrombosis that can cause subtotal or total occlusion of the lumen. Severe, acute obstruction typically results in ACS in the form of unstable angina, NSTEMI, or STEMI, depending on the level of insult.[21]
CAD is typically classified as follows (see Image. Classification of Coronary Artery Disease):
- Stable ischemic heart disease (SIHD)
- ACS
- ST-elevation myocardial infarction (STEMI)
- Non-ST elevation myocardial infarction (NSTEMI)
- Unstable angina
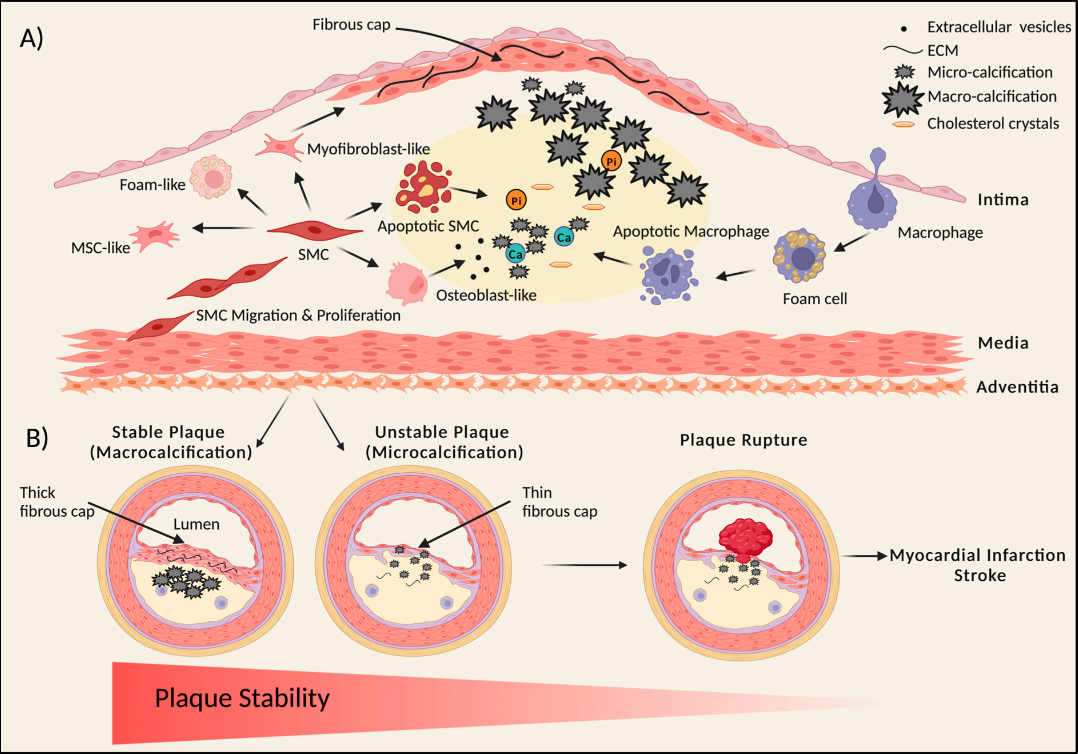
Key stages involved in atherosclerotic plaque formation include the following (see Image. Atherosclerotic Plaque Formation):
Plaque Initiation and Progression
During atherosclerosis, vascular smooth muscle cells (SMCs) proliferate and migrate, forming a fibrous cap that stabilizes the plaque. These SMCs can undergo transdifferentiation, giving rise to various cell types within the plaque core, including osteoblast-like, myofibroblast-like, foam-like, and mesenchymal-stem-like cells. As SMCs release calcifying extracellular vesicles and undergo apoptosis, small calcified deposits, known as microcalcifications, form.
Monocytes enter the thickened intima via the endothelium, absorb lipids, and transform into foam cells, which can also die and release extracellular vesicles and apoptotic bodies, further contributing to calcification. Over time, these microcalcifications develop into more significant calcium deposits, or macrocalcifications, which may evolve into calcified sheets and plates. These sheets can fracture, leading to nodular calcification, which increases the risk of plaque rupture and subsequent thrombosis. Macrocalcifications within a thin fibrous cap can also contribute to plaque rupture.
Plaque Stability and Calcification
Plaque stability is intimately linked with the type of calcification present. Stable plaques are often characterized by macrocalcifications and a thick, collagen-rich extracellular matrix within the fibrous cap, providing structural stability. In contrast, unstable plaques tend to have microcalcifications and a thin fibrous cap, heightening the risk of plaque rupture. Microcalcifications increase mechanical stress within the fibrous cap, making it more prone to rupture, potentially leading to myocardial infarction or stroke.[22]
History and Physical
Pain caused by CAD is known as angina pectoris, typically presenting as a gradual onset of discomfort in the chest, particularly behind the sternum. This discomfort can be triggered by physical exertion or emotional stress and may also occur at rest in cases of ACS. The pain often radiates to the left arm, neck, jaw, teeth, and ear, which may be explained by the convergence of signals from the vagus, trigeminal, and cervical spinal nerves (C2-C3). In addition to chest discomfort, patients frequently experience symptoms such as shortness of breath, nausea, and dizziness.
Research has shown that both men and women with CAD commonly experience chest pain, with this symptom being more prevalent than atypical presentations. Atypical pain is often described as pain in the epigastric region or back or as sensations of burning, stabbing, or indigestion. Women may complain of shortness of breath, easy fatigability, and back pain.[23]
Angina pectoris may be classified into 2 types: stable and unstable. Stable angina is generally triggered by physical activity, whereas unstable angina can occur suddenly, even at rest. Patients with unstable angina have a poorer prognosis and a higher risk of progressing to myocardial infarction. Individuals with CAD may exhibit either typical (classic) or atypical (nonclassic) pain. Classic pain generally presents as midsternal discomfort, sometimes radiating to the left neck, shoulder, or arm. However, in some cases, the only symptom may be craniofacial pain during an ischemic event.
Patients experiencing atypical pain are less likely to receive an accurate diagnosis, which is associated with a threefold increase in mortality compared to those with classic angina symptoms. Moreover, a significant proportion of myocardial infarction cases present either asymptomatically or with subtle, nonclassic symptoms, often identified incidentally during routine electrocardiography (ECG) that reveals abnormal Q waves. In some instances, patients may report pain in areas of the body other than the heart, a condition known as heterotopic pain, which can lead to misdiagnosis and unnecessary medical procedures.
Physical examination should include inspection, palpation, and auscultation. During inspection, one must look for acute distress, jugular venous distention, and peripheral edema. During palpation, one should feel a fluid thrill or heave. The severity of peripheral edema or jugular vein distension, if present, should be evaluated. During auscultation, the heart must be examined in all 4 locations, and the lungs should be assessed with a particular focus on the lower zones.
Many individuals with CAD present with nonclassic symptoms that may not be detected during standard emergency department assessments, including history taking, physical examination, and 12-lead ECG. The diagnosis may be overlooked if these patients are not admitted for further evaluation. Studies have shown that 2% to 5% of patients with myocardial infarction who are mistakenly sent home from the emergency department experience poor outcomes, making these individuals a significant source of malpractice claims in emergency medicine. Misdiagnosis often occurs due to the absence of chest pain and lack of ST-segment elevation on the ECG.[24]
Evaluation
Several modalities are highly useful in evaluating CAD, including ECG, echocardiography, chest radiography, stress testing, cardiac catheterization, and serum markers. Patient presentation dictates how these tests are utilized. Below are details on different diagnostic modalities available for the evaluation of CAD.
Electrocardiogram
ECG is a fundamental yet enormously helpful test in evaluating CAD. ECG measures electrical activity in the cardiac conduction system, measured by 10 leads attached to the skin at standardized locations. This modality provides information about the heart's anatomy and physiology. ECG tracings typically report cardiac activity on 12 leads, with each lead correlating with a specific cardiac location.
Vital information to note on an ECG tracing includes the heart's rate, rhythm, and axis, the interpretation of which can help identify ongoing acute or chronic pathologic processes. ACS may present with ST-segment and T-wave changes. ACS-related arrhythmias may also be detected. In chronic settings, ECG can provide clues to abnormalities like axis deviation, bundle branch blocks, and ventricular hypertrophy. ECG is also a cost-effective and readily available testing modality that is not user-dependent.
Notably, symptoms help patients detect the presence of a health issue. These symptoms prompt clinicians to perform ECGs, which are essential for making informed clinical decisions, such as activating the cardiac catheterization laboratory for an urgent percutaneous coronary intervention (PCI). When patients arrive at the emergency department, their condition is often undiagnosed, and the final diagnosis is only determined after thorough testing.
Emergency medical services (EMS) personnel can now perform prehospital ECGs. However, a recent study revealed that only 44.6% of patients with ACS reached the emergency department via EMS. Furthermore, only a small percentage (24.6%) of these patients experienced STEMI, and only 56.3% of individuals with STEMI called EMS. Consequently, many patients with ACS symptoms arrive at the emergency department without a diagnosis.[25]
Additionally, patients with chest pain are more likely to receive a prehospital ECG compared to those with other symptoms. Therefore, even though prehospital ECG technology is more widely available, patients who do not report chest pain are less likely to receive this diagnostic test. Including STEMI patients in studies is essential to accurately assess symptom differences and similarities between female and male patients, especially since STEMI requires time-sensitive reperfusion therapy.[26]
Echocardiography
Echocardiography is essentially cardiac ultrasound. This valuable and noninvasive mode of testing may be performed in acute, chronic, inpatient, and outpatient settings. Echocardiography detects wall motion, valvular regurgitation and stenosis, infective or autoimmune lesions, and chamber sizes in acute settings. This tool is also helpful in diagnosing acute pulmonary pathologies like pulmonary embolism. Additionally, echocardiography evaluates the pericardial cavity. In chronic settings, echocardiography can be performed to visually monitor cardiac activity and response to therapy. The test is also used outpatient as part of stress testing.
Echocardiography also has a role in therapeutics. For example, pericardiocentesis may be performed with the needle guided by ultrasound. This test is user-dependent and often more expensive than ECG.[27]
Until recently, the evaluation of regional wall motion abnormalities was the primary focus of stress echocardiography. The European Society of Cardiology advocates the advanced ABCDE protocol, and this assessment remains the core component of step A. However, the protocol has expanded to include additional assessments.
Regional perfusion using ultrasound contrast agents may also be evaluated in step A. Step B evaluates diastolic function and pulmonary B-lines, while step C assesses left ventricular contractile and preload reserve using volumetric echocardiography. Step D focuses on Doppler-based coronary flow velocity reserve in the left anterior descending coronary artery, and step E measures ECG-based heart rate reserve in a nonimaging format. These 5 biomarkers are integrated within the ABCDE protocol, providing a comprehensive approach to risk stratification for patients with chronic coronary syndromes.[28]
Stress Test
The stress test is a relatively noninvasive test for CAD. This modality is used in the setting of suspected angina or angina equivalent and is helpful in identifying coronary pathology when interpreted in the appropriate setting. The heart is artificially exposed to stress during the test. The test is aborted if the patient develops anginal symptoms or abnormal ECG changes, particularly in ST segments, and CAD is diagnosed. ECGs are obtained before, during, and after the procedure, and the patient is continuously monitored for any symptoms.
The stress echocardiography laboratory utilizes various methods, such as exercise, dobutamine, vasodilators like dipyridamole or adenosine, and external pacing for patients with permanent pacemakers. This range of stress-inducing techniques allows for selecting the most suitable test tailored to each patient's needs. In exercise stress tests, the patient has to run on a treadmill until 85% of the age-predicted maximal heart rate is achieved. The test is aborted if a patient develops exertional hypotension, hypertension (>200/110 mm Hg), ST-segment elevations or depression, or ventricular or supraventricular arrhythmias.[29]
Chest Radiography
Chest radiographs are an essential component of the initial evaluation of cardiac disease. The standard imaging films include standing posteroanterior and left lateral decubitus views. Anteroposterior projection is occasionally obtained, especially in inpatient settings, with the patient lying down. However, this interpretation of anteroposterior films is significantly limited. Proper analysis of posteroanterior and anteroposterior views provides valuable and cost-effective information about the heart, lungs, and vasculature. Interpretation should be completed stepwise so that important information is not overlooked.
Serum Markers
Blood work aids in establishing the diagnosis and assessing therapeutic responses. In acute settings, cardiac enzymes and B-type natriuretic peptides (BNP) are often taken along with complete blood counts and metabolic panels. BNP provides information about volume overload of cardiogenic origin. However, this marker has limitations. BNP can be falsely elevated in kidney disease and falsely low in obesity.
Cardiac enzymes like creatine kinase and troponin provide information about an acute ischemic event. In chronic settings, lipid panels offer important prognostic information. C-reactive protein and erythrocyte sedimentation rate aid in assessing diseases like acute pericarditis. Liver function tests may be taken to evaluate for an infiltrative process that can affect the liver and heart simultaneously, like hemochromatosis. Liver tests also assess increased right heart pressures, especially in chronic settings.
Cardiac Catheterization
Cardiac catheterization is the gold standard and most accurate modality for evaluating ischemic coronary heart disease. Coronary angiography is used to assess the type and number of affected vessels and the severity of stenosis, which are essential in determining the appropriate approach for coronary intervention. However, this invasive procedure has potentially serious complications, and not everyone is a candidate for it.
Patients with intermediate pretest probability for CAD are suitable cardiac catheterization candidates in non-ACS settings. If ACS is diagnosed, all patients with STEMI and selected individuals with NSTEMI should receive an emergent cardiac catheterization. This procedure is performed in a cardiac catheterization laboratory. It is expertise-dependent and accomplished under moderate sedation. Cardiac catheterization requires contrast exposure, which may cause severe allergic reactions and kidney injury.[30]
As an indicator of clinical flow impairment, the extent of stenosis in a lesion is assessed by comparing the maximum stenotic diameter to the diameter of adjacent, typically normal, arterial segments. Coronary flow reduction starts with stenosis exceeding 50% and accelerates once it surpasses 70%. Measuring the percent diameter of stenosis is clinically valuable for evaluating obstruction to blood flow, especially for stenoses below 50% or above 70%.[31]
Coronary Calcium Score and Computed Tomography Angiography
Between 2013 and 2020, 10,857 patients at Haukeland University Hospital in Norway underwent coronary artery calcium (CAC) scoring and coronary computed tomography angiography (CCTA). Obstructive CAD was diagnosed on CCTA if 1 or more vessels with at least 50% stenosis were identified. High-risk CAD included obstructive stenoses in the left main stem, proximal left anterior descending artery, or all 3 major coronary territories, with involvement of at least 1 proximal segment.
A CAC score of 0 in symptomatic patients effectively excluded obstructive CAD and high-risk CAD in 98.2% and 99.4% of cases, respectively. This extensive registry-based study supports using CAC testing for initial patient assessment and as a preliminary step before further cardiac evaluation. However, a full CCTA might be necessary in patients younger than 45 to rule out obstructive CAD safely.[32]
Treatment / Management
CAD could present either as SIHD or ACS. The management depends on the particular disease type, as discussed below.
Stable Ischemic Heart Disease
SIHD is a chronic condition that primarily presents as stable angina, manifesting with substernal chest pain or pressure that worsens with exertion or emotional stress and gets relieved with rest or nitroglycerin treatment. Duration is at least 2 months. Note that classic anginal symptoms could be absent in some patients, and SIHD may manifest with atypical symptoms and exertional dyspnea in certain demographic groups, including women, older individuals, and people with diabetes mellitus. Management of SIHD includes both nonpharmacologic and pharmacologic interventions. Lifestyle modifications include smoking cessation, regular exercise, weight loss, reasonable control of diabetes and hypertension, and a healthy diet. Pharmacologic interventions include cardioprotective and antianginal medications.
Every patient should get guideline-directed medical therapy (GDMT), which includes low-dose aspirin, β-blocker, as-needed nitroglycerin, and moderate-to-high-intensity statin. If symptoms are not controlled despite GDMT, β-blocker therapy should be titrated up to achieve heart rates between 55 and 60. Adding calcium channel blockers and long-acting nitrates should also be considered.[33] Ranolazine may also be added to relieve refractory anginal symptoms. Failure of maximal GDMT to relieve angina warrants cardiac catheterization to visualize coronary anatomy. A decision on PCI or coronary artery bypass graft (CABG) should also be made based on patient profile.[34]
Acute Coronary Syndrome
ACS presents as sudden-onset substernal chest pain or pressure, typically radiating to the neck and left arm, and may be accompanied by dyspnea, palpitations, dizziness, syncope, cardiac arrest, or new-onset congestive heart failure. Prompt ECG testing is necessary for all patients with ACS to assess for STEMI. EMS personnel may obtain an ECG in the prehospital setting. STEMI is recognized by ST elevation in contiguous leads of 1 mm in limb or precordial leads except V2 and V3. A STEMI diagnosis in leads V2 and V3 requires 2-mm elevations in men and 1.5-mm elevations in women.
A new-onset left bundle branch block (LBBB) is considered a STEMI equivalent. A STEMI warrants an emergency PCI, preferably performed in a PCI-capable facility within 2 hours. If the PCI-capable facility is more than 2 hours away, intravenous thrombolytic therapy is indicated after ensuring no contraindications.
A true STEMI must be differentiated from mimics (based on ECG), such as acute pericarditis, Brugada syndrome, early repolarization, and left ventricular hypertrophy-associated changes. Upon presentation, all patients should get a full dose of sublingual aspirin (324 mg). Nitrates should be given for pain relief after ensuring that the patient has no contraindications, such as hypotension, right ventricular failure, and intake of phosphodiesterase inhibitors in the past 24 to 48 hours. High-dose statin therapy and β-blockers should also be initiated early. P2Y12 inhibitors (prasugrel, ticagrelor, or prasugrel) should be considered based on patient profile.
Individuals with non-ST-elevation ACS should receive anticoagulation, typically with heparin or enoxaparin. For NSTEMI, early invasive therapy within 24 hours is advised for patients with intermediate-to-high TIMI scores (>2).[35][36]
Regular visits with cardiologists and family physicians are critical to the long-term management of CAD. Medication adherence and lifestyle modification are also essential.
A systematic review following stenting conducted for the 2016 Americal College of Cardiology/AHA DAPT guidelines analyzed the outcomes of death, major bleeding, myocardial infarction, stent thrombosis, and major adverse cardiac events in 33,051 patients across 11 randomized controlled trials. These trials compared prolonged DAPT to short-course DAPT as a regimen following a drug-eluting stent (DES) procedure and as an agent for secondary prevention after a myocardial infarction episode. The review found that, in patients treated with DES, extended DAPT reduced the incidence of stent thrombosis and myocardial infarction but led to an increased risk of significant bleeding. Additionally, patients with a history of myocardial infarction showed a reduction in cardiovascular events, although this benefit came with a higher risk of bleeding.[37]
Several trials have provided evidence supporting DAPT for 6 months, followed by continued aspirin use beyond this period. The largest of these trials, ISAR-SAFE (a randomized, double-blind trial of 6 versus 12 months of DAPT after DES implantation), involved 4,005 patients, 60% of whom had stable CAD. In these studies, patients continued taking aspirin throughout the 12-month trial period.[38]
However, data is limited on whether aspirin or clopidogrel is the preferable antiplatelet agent for indefinite use after the initial 12 months post-PCI. The HOST-EXAM (Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases-Extended Antiplatelet Monotherapy) trial, which included 5,530 East Asian participants who had tolerated DAPT for 6 to 18 months without ischemic or major bleeding complications, compared the effects of continuing with either clopidogrel (75 mg daily) or aspirin (100 mg daily) for an additional 24 months.[39]
Differential Diagnosis
CAD has a wide range of differential diagnoses because of the heart's proximity to adjacent organs, including the lungs, stomach, prominent vessels, and musculoskeletal organs. Acute anginal chest pain can mimic acute pericarditis, myocarditis, Prinzmetal angina, pericardial effusion, acute bronchitis, pneumonia, pleuritis, pleural effusion, aortic dissection, gastroesophageal reflux disease, peptic ulcer disease, esophageal motility disorders, and costochondritis. SIHD may also mimic gastroesophageal reflux disease, peptic ulcer disease, costochondritis, and pleuritis. History taking, physical examination, and diagnostic studies should be diligently conducted to narrow the differential diagnosis and reach an accurate diagnosis.
Toxicity and Adverse Effect Management
Both medical and surgical management for IHD have potential side effects and complications. Careful selection, physician expertise, and patient education can mitigate these undesirable effects. Aspirin therapy may cause bleeding, idiosyncratic, and allergic drug reactions.[40] Statin therapy may produce myalgias, diarrhea, and arthralgia.[41]
β-blocker use can result in bradycardia and hypotension. Angiotensin-converting enzyme inhibitor intake may result in hypotension, dizziness, creatinine elevation, cough, and allergic reactions, including angioedema.[42] PCI can cause coronary artery perforation and stent thrombosis acutely and instent restenosis chronically.[43] CABG's potential complications include but are not limited to arrhythmias, cardiac tamponade, postoperative bleeding, infection, renal impairment, and phrenic nerve injury.
Prognosis
CAD's prognosis depends on multiple factors, some of which are modifiable. The patient's age, gender, family history and genetics, ethnicity, dietary and smoking habits, medication compliance, availability of healthcare and financial status, and the number of arteries involved are some factors. Comorbid conditions, including diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease, also have a role in the overall outcome.[44]
Complications
Arrhythmias, congestive heart failure, mitral regurgitation, mechanical complications, pericarditis, aneurysm formation, and mural thrombi are the main complications associated with CAD.[45][46] These complications are discussed below.
Arrhythmias
Numerous arrhythmic complications can arise in the early period following a myocardial infarction episode. The appropriate management of these arrhythmias depends on several patient-specific factors, including the timing of symptom onset, left ventricular function, overall clinical condition, and the presence of comorbidities.
Certain peri-infarct arrhythmias, such as ventricular arrhythmias, atrial fibrillation, and persistent high-grade atrioventricular block, necessitate immediate intervention. Other arrhythmias, such as sinus bradycardia and tachycardia and transient high-grade atrioventricular block, may require urgent treatment but often resolve on their own following reperfusion and as time progresses. Recognizing these arrhythmias, understanding their likelihood, and being aware of the associated risks are crucial for improving preparedness and outcomes in these vulnerable patients.[47]
Congestive Heart Failure
Investigating cardiac biomechanics is crucial for evaluating the overall cardiovascular function in patients with myocardial infarction, as this population is at a heightened risk of developing chronic heart failure (CHF) due to pathological left ventricular remodeling. Monitoring left ventricular ejection fraction (LVEF) is particularly important in managing CHF, as a reduced LVEF is linked to a poor prognosis, diminished quality of life, and higher mortality and hospitalization rates.
An LVEF decline during follow-up is a clear indicator of CHF progression. A decreased LVEF, signaling the transition from heart failure with preserved ejection fraction (HFpEF) to heart failure with mid-range ejection fraction (HFmrEF) or reduced ejection fraction (HFrEF), is typically seen as a sign of CHF progression in the postinfarction period. Additionally, many studies have identified heart rate variability as a reliable predictor of high mortality risk and CHF progression.[48]
Mitral Regurgitation
A study analyzed 1,000 patients admitted with acute myocardial infarction (AMI) from 2016 to 2017, all of whom underwent PCI followed by predischarge transthoracic echocardiography. Mitral regurgitation was identified in 294 patients (29%) and was categorized as mild in 224 cases (76%), moderate in 61 cases (21%), and severe in 9 cases (3%). Compared to patients without mitral regurgitation, people with this pathology were older (70 ± 12 years vs. 63 ± 13 years; p < 0.001) and exhibited worse LVEF (52 ± 15% vs. 55 ± 11%; p < 0.001) and lower creatinine clearance (69 ± 33 ml/min vs. 90 ± 39 ml/min; p < 0.001).
Additionally, patients with mitral regurgitation had higher rates of hypertension (64% vs. 55%; p = 0.012), heart failure (3.4% vs. 1.1%; p = 0.014), prior myocardial infarction (28% vs. 20%; p = 0.005), and significant flow-limiting lesions in the circumflex artery (50% vs. 33%; p < 0.001) or right coronary artery (51% vs. 42%; p = 0.014). The prevalence and severity of mitral regurgitation were consistent across different AMI subtypes.[49]
Mechanical Complications
During the 13-year study period from 2003 to September 2015, 3,982,655 hospitalizations for STEMI and 5,143,707 hospitalizations for NSTEMI were analyzed. The incidence of mechanical complications, such as papillary muscle rupture (PMR), ventricular septal defects (VSDs), and ventricular free wall rupture (FWR), both overall and individually, remained relatively stable over time in the STEMI and NSTEMI groups.
In the STEMI group, 10,726 patients (0.27%) experienced mechanical complications, including 2,024 cases (0.05%) of PMR, 8,401 cases (0.21%) of VSDs, and 301 cases (0.01%) of FWR. The in-hospital mortality rate for patients with STEMI with mechanical complications was 42.4%. In the NSTEMI group, 3,041 patients (0.06%) experienced mechanical complications, with 628 cases (0.01%) of PMR, 1,943 cases (0.04%) of VSDs, and 470 cases (0.01%) of FWR. The in-hospital mortality rate for patients with NSTEMI with mechanical complications was 25%.[50]
Pericarditis
Postmyocardial infarction pericarditis (PMIP) is an uncommon complication of STEMI in the era of coronary reperfusion. PMIP worsens short-term outcomes but does not affect long-term prognosis. The condition is often associated with larger infarcts.
A retrospective analysis of 6,282 patients with STEMI from the Acute Coronary Syndrome Israeli Survey (2000-2013) found that 76 patients (1.2%) had PMIP. Individuals with PMIP tended to be younger (median age 58 vs. 61; p = 0.045), showed lower rates of hypertension, had elevated cardiac biomarkers, and more frequently had reduced left ventricular ejection fraction (87% vs. 67%; p = 0.001). Patients with PMIP also experienced longer times to reperfusion (225 minutes vs. 183 minutes; p = 0.016) and extended hospital stays (7 vs. 5 days; p < 0.001). However, no significant differences in survival rates were observed at 30 days, 1 year, and 5 years.[51]
Aneurysm Formation
Data on the prevalence and outcomes of left ventricular aneurysms following AMI are limited. A retrospective cohort analysis, using the National Inpatient Sample from 2000 to 2017, evaluated AMI admissions for the presence of left ventricular aneurysms and associated complications, such as ventricular arrhythmias, mechanical complications, cardiac arrest, pump failure, left ventricular thrombus, and stroke. The study reviewed 11,622,528 AMI admissions, of which 17,626 (0.2%) had left ventricular aneurysms.
Patients with left ventricular aneurysms were more frequently female, had higher rates of comorbidities, and were more often admitted to large urban hospitals (all p < 0.001). From 2000 to 2017, a slight increase was observed in the prevalence of left ventricular aneurysms among patients with and without acute STEMI (p < 0.001). Left ventricular aneurysms were more commonly observed with anterior STEMI (31%) compared to inferior (12.3%) and other types (7.9%).[52]
Mural Thrombi
Information on the prevalence of ventricular mural thrombus following AMI is limited. In a study involving 50 patients admitted to a coronary care unit for acute anterior myocardial infarction with ST-segment elevation, coronary arteriography, left ventricular angiography, and revascularization were performed. Transthoracic echocardiography was utilized to evaluate the left ventricular chamber using both fundamental and second harmonic imaging along with contrast agents.
A left ventricular mural thrombus was confirmed in 12% (6 patients) of cases. Among patients with time-to-revascularization exceeding 3 hours, 35% had a left ventricular mural thrombus, compared to none in patients with a time-to-revascularization of 3 hours or less (p = 0.003). Contrast echocardiography emerged as the most accurate method for detecting left ventricular mural thrombus, regardless of the clinician's experience level.[53]
Deterrence and Patient Education
A combination of modifiable and nonmodifiable factors causes CAD. Primary care providers should focus on modifiable risk factor modification on each routine visit. Tight control of diabetes, hypertension, and lipid levels, in addition to smoking cessation, weight loss, and exercise, can make a huge difference. Since CAD is a global public health concern, increasing awareness through school curricula and various media outlets is essential.
Pearls and Other Issues
Several landmark trials have been conducted over the past few decades, and they have changed how we care for patients with CAD. Individual trial results are beyond this article's scope. However, some critical studies include ISIS-2, CURE, CLARITY-TIMI 28, TIRTON-TIMI 38, PLATO, and CURRENT-OASIS 7, which were conducted to help determine guidelines for antiplatelet medications.[54] SYNERGY, ACUITY, ExTRACT-TIMI 25, OASIS-5, and ATLAS ACS/TIMI 52 evaluated the value of anticoagulation in CAD management. ADMIRAL, ACUITY, ISAR-REACT 3, and HORIZONS-AMI were famous trials that investigated GpIIb/IIIa use. COMMIT studied β-blockers, while SHOCK, DANAMI-2, BASKET-LATE, TIMACS, and BASKET-PROVE explored PCI and CABG.
The MIRACL trial investigated statin use. Patients with diabetes or low HDL cholesterol are at a higher risk and gain more significant absolute benefit from early treatment with 80 mg of atorvastatin daily compared to those without these risk factors. In the overall population of the MIRACL study, administering 80 mg of atorvastatin resulted in a 2.6% reduction in the absolute risk of experiencing a primary endpoint event, such as death, nonfatal myocardial infarction, resuscitated cardiac arrest, and recurrent ischemia, compared to placebo. For patients with diabetes or low HDL cholesterol, the reduction in absolute risk was even more significant, at 4.4% and 3.7%, respectively.[55]
Enhancing Healthcare Team Outcomes
IHD frequently presents a diagnostic dilemma. Patients can present with nonspecific symptoms like chest pain or shortness of breath, which may arise from a myriad of diseases, including gastrointestinal, cardiac, musculoskeletal, psychological, and pulmonary causes. While a cardiologist is often a central player in IHD evaluation, taking other team members on board as indicated is essential, including a gastroenterologist, pulmonologist, and psychiatrist. Radiologists are also a vital resource in the whole process. Nurses are an integral part of the diagnostic and therapeutic workup and provide critical bedside information not witnessed by the physicians in their short encounters.