Introduction
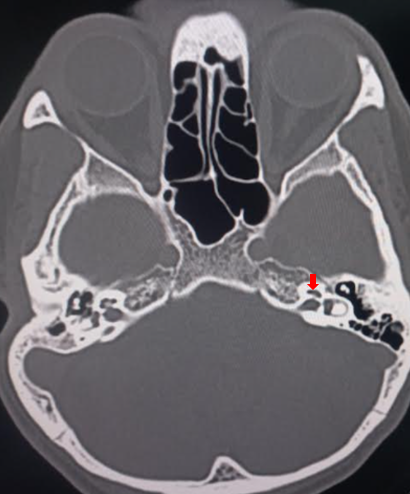
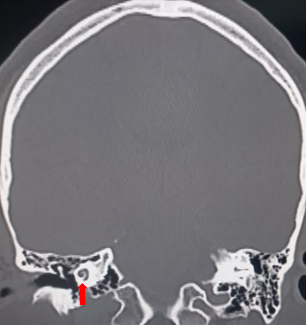
The cochlea is a fluid-filled, spiral-shaped cavity found in the inner ear that plays a vital role in the sense of hearing and participates in the process of auditory transduction. Sound waves are transduced into electrical impulses that the brain can interpret as individual sound frequencies. The spiral configuration of the cochlea allows for differing frequencies to stimulate specific areas along the spiral, which results in a tonotopic map that enables humans to perceive various frequencies of sound. See Images. Cochlea, Computed Tomography, Cochlea, Coronal Computed Tomography.
Specific areas along the cochlea are stimulated by vibrations carried within a fluid known as endolymph in the cochlear duct. The vibrations are then converted to electrical impulses in the cochlear duct through mechanical stimulation of hair cells within a unique structure known as the organ of Corti. The vestibulocochlear nerve (CN VIII) then carries these impulses from the cochlea to the brain's auditory cortex for interpretation.[1]
Issues of Concern
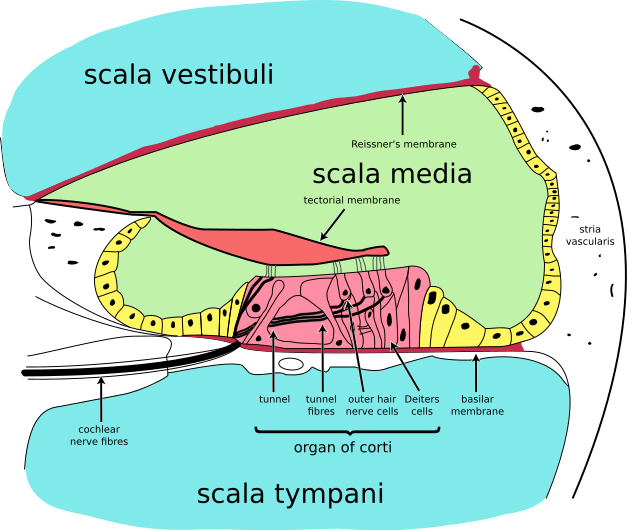
Understanding cochlear anatomy is essential to understanding its physiology. The inner ear is comprised of a bony and membranous labyrinth. The bony labyrinth is a cavity within the temporal bone consisting of the vestibule, semicircular canals, and cochlea. The cochlear tube is formed by three membranous and fluid-filled chambers that run parallel to each other; the scala vestibuli (SV) or vestibular duct, the scala media (SM) or cochlear duct, and the scala tympani (ST) or tympanic duct. The SV and ST are filled with perilymph, while the SM is filled with endolymph. See Image. Cochlea, Cross Section.
The composition of perilymph resembles that of extracellular fluid, with sodium as the primary cation, and, via the perilymphatic duct, serves as a connection to the CSF of the subarachnoid space. The perilymph contains proteins, such as enzymes and immunoglobulins, which are important for metabolism and the immune response.[2]
The composition of endolymph resembles that of intracellular fluid, with potassium as the primary cation. As there is a higher potassium concentration than sodium, the endolymph's electrical potential is approximately 80-90 mV more positive than perilymph.[3] This electrical potential difference allows potassium ions to flow into the hair cells during mechanical stimulation of the hair bundle—discussed later in further detail. The stria vascularis is highly vascularized tissue that lines the lateral walls of the cochlea and is responsible for producing the endolymph for the SM and maintaining the ion balance surrounding the outer and inner hair cells of the organ of Corti.[4]
The helicotrema is the apex of the cochlea and is where the ST and SV meet.[5] The Reissner membrane is what separates the SV from the SM. The spiral ligament (SL), connected to the Reissner membrane and the basilar membrane (BM), forms the outer wall of the scala media. The SL contains blood vessels and fibrocytes, which play an important role in immune response and ion homeostasis.[6] The osseous spiral lamina and BM separate the SM from the ST, with the BM determining the mechanical wave propagation properties.[7]
The organ of Corti is a cellular layer sitting on top of the BM in which sensory hair cells are found. As previously mentioned, these sensory hair cells are activated by the potential difference between the perilymph and the endolymph. These hair cells are topped with hair-like structures called stereocilia. There is one single row of inner hair cells (IHC) and three rows of outer hair cells (OHC), separated by the tunnel of Corti.[8] Sound waves cause vibrations, which bend the hair cell stereocilia via an electromechanical force, leading to electrical impulses.[9] These electrical impulses travel to the brain via the vestibulocochlear nerve (CN VIII).
Cellular Level
Hair cells are specialized cells that play an essential role in cochlear function. Hair cells are found within the organ of Corti and are divided into inner hair cells (IHCs) and outer hair cells (OHCs). IHCs are the true auditory receptor cells that synapse with bipolar spiral ganglion neurons to send afferent nerve impulses back to the brain via the cochlear nerve. Actin filaments connect the stereocilia at the tips of the IHCs, and mechanically gated potassium channels open in response to vibration leading to afferent cochlear nerve signaling.[10]
Approximately 95% of spiral ganglion neurons synapse on IHCs. The remaining 5-10% of spiral ganglion neurons innervate the OHCs. The OHCs increase the maximum amplitude of the traveling wave of vibration. Efferent nerve fibers from the brain synapse on OHCs and decrease their ability to enhance the amplitude of the vibrational wave.[11]
Development
The inner ear develops embryologically from ectodermal cells by week 4 of gestation. Invagination of these cells forms the otic vesicle, which contains a dorsal and a ventral pouch. The dorsal pouch forms the endolymphatic duct and vestibular structures, while the ventral pouch elongates to become the cochlea. The cochlea forms a two-and-a-half turn coil by week 10 of gestation and reaches a maximum labyrinth length by 18 weeks. The organ of Corti develops from the sensory neuroepithelium within the cochlear duct. SOX2 is an important transcription factor that plays a key role in cochlear development. Mutations in SOX2 have been shown to be associated with sensorineural hearing loss.[10]
Function
The ultimate goal of the cochlea is to transform complex airborne vibrations into auditory neural impulses. The tonotopic map created by the spiral of the cochlea enables individuals to simultaneously interpret a vast amount of sounds through vibrations carried from the perilymph to the endolymph in the cochlear duct. The anatomy of the cochlea allows it to efficiently carry vibrations that are ultimately converted to electrical impulses and interpreted by the brain's auditory cortex.[11]
Mechanism
The process of auditory transduction begins with sound waves entering the external acoustic meatus and striking the tympanic membrane, resulting in vibration. These vibrations are then transferred to the middle ear along the ossicular chain of the malleus, incus, and stapes. The footplate of the stapes contacts the oval window in a piston-like motion that transmits vibration to perilymph within the cochlea. The vibrations then travel up the cochlea to the apex through a hollow bony tube called the scala vestibuli and then from the apex to the base of the cochlea through another hollow, bony tube called the scala tympani.[10]
At the end of the scala tympani, the vibrations within the perilymph displace the round window. The cochlear duct lies between the scala vestibuli and the scala tympani. This hollow, bony tube contains endolymph, which has a higher positive potential than the surrounding perilymph. This positive potential results from high potassium and low sodium ions concentrations compared to the surrounding perilymph.[12] The Reissner membrane maintains the differences in ion concentration between the endolymph and perilymph. The Reissner membrane separates the scala vestibuli from the cochlear duct and the stria vascularis, specialized cells lining the lateral wall of the cochlear duct. The structure separating the scala tympani from the cochlear duct is known as the basilar membrane. The basilar membrane contains a specialized structure known as the organ of Corti that plays a key role in auditory transduction.
The organ of Corti contains hair cells that respond to vibration by brushing their stereocilia against a fixed structure called the tectorial membrane. The result of the hair cells bending against the tectorial membrane is the depolarization of the attached nerve fibers. The frequency of the vibration traveling through the perilymph will correspond to an area along the BM of the cochlea that is maximally stimulated. The BM of the cochlea is not uniform in its width or stiffness. At the base of the cochlea, the BM is more narrow and stiff, while at the apex of the cochlea, the BM is wider and more flexible. Additionally, the further away from the base of the cochlea, the elasticity of the BM decreases; the BM is most elastic at the apex of the cochlea.
This non-uniformity of the BM creates a tonotopic map and enables humans to hear different sound frequencies. For example, an auditory vibration will stimulate a specific location on the BM, depending on the sound frequency. The highest frequencies will be registered at the base of the cochlea, while the lower frequencies will be registered at the apex.[13]
Related Testing
Testing to determine if the cochlea or related structures are involved in hearing loss begins with the Rinne and Weber tests. These tests are accomplished using a 512 Hz tuning fork to determine if the cause of hearing loss is conductive or sensorineural. Conductive hearing loss etiologies include more mechanical dysfunctions in the outer and middle ear, such as cerumen impaction, otitis media, and damage to the ossicles or tympanic membrane. Sensorineural hearing loss involves damage to the specialized nervous system that makes up the inner ear, the cochlea or nerves exiting the cochlea.
The Weber test is performed by striking the 512 Hz tuning fork and placing it on the center of the head. The patient is then asked if the sound is lateralized to one or both ears. The sound will lateralize to the dysfunctional ear with conductive hearing loss and to the functioning ear with sensorineural hearing loss.[14]
Next, the Rinne test is performed by striking the 512 Hz tuning fork and placing it on the mastoid process of each ear. The patient is instructed to indicate when the sound is no longer heard, at which point the tuning fork is moved to the auditory meatus, and the patient is instructed to repeat the process. Normal auditory function will have a 2:1 ratio of bone-to-air conduction times. If the hearing loss is conductive, bone conduction is heard longer than or equal to air conduction. If the hearing loss is sensorineural, air conduction is heard longer than bone conduction but less than the normal 2:1 ratio.[15]
Electrocochleography is a test that records the electrical potentials generated by the cochlea and the vestibulocochlear nerve (CN VIII). An electrode is placed in the ear canal, on the tympanic membrane, or through the tympanic membrane on the cochlea. It measures the summating potential generated by hair cells of the cochlea and the action potentials of the cochlear nerve in response to acoustic stimulation. Electrocochleography is most useful in diagnosing endolymphatic hydrops, commonly seen in Ménierè disease, and for intraoperative monitoring of the cochlea and CN VIII.[16]
Pathophysiology
As previously mentioned, defects in the outer ear lead to conductive hearing loss, while defects in the inner ear lead to sensorineural hearing loss, the most common type of hearing impairment worldwide. Sensorineural hearing loss arises due to damage to the cochlea or auditory nerve, and several structures are often affected simultaneously. There are many causes of sensorineural hearing loss, including genetic mutations affecting the structures of the inner ear and environmental insults such as noise, ototoxic substances, and hypoxia.
Clinical diagnosis of sensorineural hearing loss is most commonly accomplished by measuring detection thresholds and comparing these to normative values to determine the degree of hearing loss. In addition to causing decreased sensitivity to weak sounds, sensorineural hearing loss has several adverse perceptual consequences, including loudness recruitment, poor perception of pitch and auditory space, and difficulty understanding speech, particularly in the presence of background noise. The condition is usually incurable, with treatment focusing on restoring the audibility of sounds made inaudible by hearing loss using either hearing aids or cochlear implants.[17]
The two main etiologies of sensorineural hearing loss specific to cochlear dysfunction include noise damage and Ménierè disease. Noise damage occurs during brief or sustained exposure to extremely loud noise, with prolonged or repeated exposure to sounds of greater than 85 decibels increasing the risk of permanent cochlear damage. The damage associated with loud noise exposure destroys the hair cells in the cochlea, which leads to an inability to stimulate afferent nerves and, therefore, a failure to hear various frequencies.[18]
Ménierè disease is a slowly progressive inner ear disorder characterized by vertigo, tinnitus, and hearing loss. It is caused by endolymphatic hydrops, or the accumulation of endolymphatic fluid within the cochlea, causing distention. Ménierè disease involves dysregulation of aquaporin channels and osmotic disequilibrium involving endolymph production from perilymph through the Reissner membrane and stria vascularis.[19]
Alport syndrome is a genetic defect of type IV collagen, which is present in the basement membrane of the eyes, kidneys, and cochlea. It commonly presents as glomerulonephritis, decreased visual acuity, and bilateral sensorineural hearing loss.[20]
Clinical Significance
A cochlear implant is a device that uses electricity to directly stimulate the spiral ganglion cells of the auditory nerve, bypassing the damaged hearing apparatus and restoring hearing in those with sensorineural hearing loss. A cochlear implant electrode is placed through the round window and implanted in the scala tympani. The electrode provides direct stimulation to the auditory nerve, allowing signals to be sent to the auditory cortex of the brain. Of course, for a cochlear implant to be effective, there must be relatively preserved anatomy of the cochlea and CN VIII.[21]
It is well known that many loop diuretics can be ototoxic, as they inhibit the Na/K/2Cl cotransporter in the stria vascularis. Loop diuretics induce pathological changes in the cochlea, including the formation of edematous spaces in the stria vascularis epithelium. This then leads to decreased cochlear electrical potential and, with long-term use, temporary or permanent sensorineural hearing loss.[22]
From a clinical perspective, dysfunction of normal cochlear physiology will manifest as hearing loss. Cochlear dysfunction should be in the clinician's differential diagnosis for any patient presenting with hearing loss.[10]