Continuing Education Activity
Cirrhosis is characterized by fibrosis and nodule formation of the liver secondary to chronic injury, leading to alteration of the normal lobular organization of the liver. Various insults can injure the liver, including viral infections, toxins, hereditary conditions, or autoimmune processes. With each injury, the liver initially forms scar tissue (fibrosis) without losing its function. After a chronic injury, most of the liver tissue becomes fibrotic, leading to loss of function and the development of cirrhosis. This activity reviews the causes, evaluation, and management of hepatic cirrhosis and highlights the interprofessional team's role in managing patients with this condition.
Objectives:
Identify the pathophysiology of cirrhosis.
Assess the etiology of cirrhosis.
Evaluate the presentation of a patient with cirrhosis.
Communicate the importance of improving care coordination amongst interprofessional team members to improve outcomes for cirrhotic patients.
Introduction
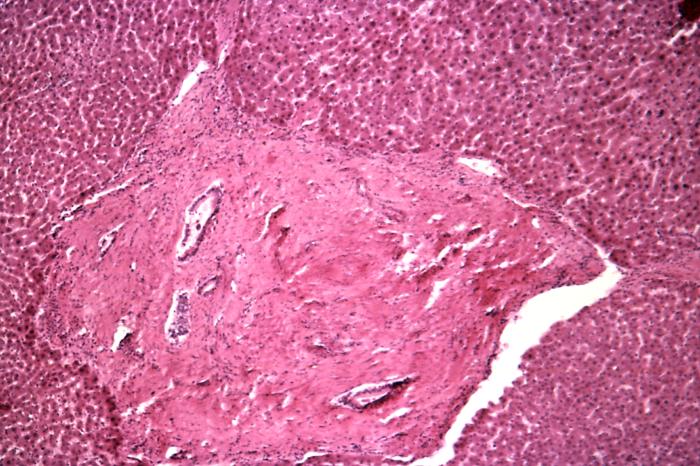
Cirrhosis is characterized by fibrosis and nodule formation of the liver, secondary to a chronic injury, which leads to alteration of the normal lobular organization of the liver. Various insults can injure the liver, including viral infections, toxins, hereditary conditions, or autoimmune processes. The liver initially forms scar tissue (fibrosis) with each injury without losing its function. After a long-standing injury, most of the liver tissue gets fibrosed, leading to loss of function and the development of cirrhosis. See image. Cirrhosis, Liver.
Etiology
Chronic liver diseases usually progress to cirrhosis. In the developed world, the most common causes of cirrhosis are hepatitis C virus (HCV), alcoholic liver disease, and nonalcoholic steatohepatitis (NASH). In contrast, hepatitis B virus (HBV) and HCV are the most common causes in the developing world.[1] Other causes of cirrhosis include autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, Wilson disease, alpha-1 antitrypsin deficiency, Budd-Chiari syndrome, drug-induced liver cirrhosis, and chronic right-sided heart failure. Cryptogenic cirrhosis is defined as cirrhosis of unclear etiology.
Epidemiology
The worldwide prevalence of cirrhosis is unknown; however, it has been estimated to be between 0.15% and 0.27% in the United States.[2][3]
Pathophysiology
Multiple cells play a role in liver cirrhosis, including hepatocytes and sinusoidal lining cells such as hepatic stellate cells (HSCs), sinusoidal endothelial cells (SECs), and Kupffer cells (KCs). HSCs form a part of the wall of the liver sinusoids, and their function is to store vitamin A. When these cells are exposed to inflammatory cytokines, they get activated, transform into myofibroblasts, and start depositing collagen, which results in fibrosis. SECs form the endothelial lining and are characterized by the fenestrations they make in the wall that allow the exchange of fluid and nutrients between the sinusoids and the hepatocytes.[4] Defenestration of the sinusoidal wall can happen secondary to chronic alcohol use and promote perisinusoidal fibrosis.[5] KCs are satellite macrophages that line the wall of the sinusoids as well. Studies from animal models have shown that they play a role in liver fibrosis by releasing harmful mediators when exposed to injurious agents and acting as antigen-presenting cells for viruses.[6] Hepatocytes are also involved in cirrhosis's pathogenesis, as damaged hepatocytes release reactive oxygen species and inflammatory mediators that can promote activating HSCs and liver fibrosis.[7]
The major cause of morbidity and mortality in cirrhotic patients is the development of portal hypertension and hyperdynamic circulation. Portal hypertension develops secondary to fibrosis and vasoregulatory changes intrahepatically and systematically, leading to collateral circulation formation and hyperdynamic circulation.[8] Intrahepatically, SECs synthesize nitric oxide (NO) and endothelin-1 (ET-1), which act on HSCs, causing relaxation or contraction of the sinusoids, respectively, and controlling sinusoidal blood flow. In patients with cirrhosis, there is an increase in ET-1 production and the sensitivity of its receptors with a decrease in NO production. This leads to increased intrahepatic vasoconstriction and resistance, initiating portal hypertension. Vascular remodeling mediated by the contractile effects of HSCs in the sinusoids augments the increase in vascular resistance. To compensate for this increase in intrahepatic pressure, collateral circulation is formed.[8] In systemic and splanchnic circulation, the opposite effect happens, with an increase in NO production, leading to systemic and splanchnic vasodilation and decreased systemic vascular resistance. This activates the renin-angiotensin-aldosterone system, leading to sodium and water retention and hyperdynamic circulation. Thus, in cirrhosis with portal hypertension, there is a depletion of vasodilators (predominantly NO) intrahepatic-ally but a renin-excess of NO extrahepatically in the splanchnic and systemic circulation, leading to sinusoidal vasoconstriction and splanchnic (systemic) vasodilation. The collaterals also contribute to the hyperdynamic circulation by increasing the venous return to the heart.[8][9]
Histopathology
Cirrhosis is classified based on morphology or etiology.
-
Morphology Classification
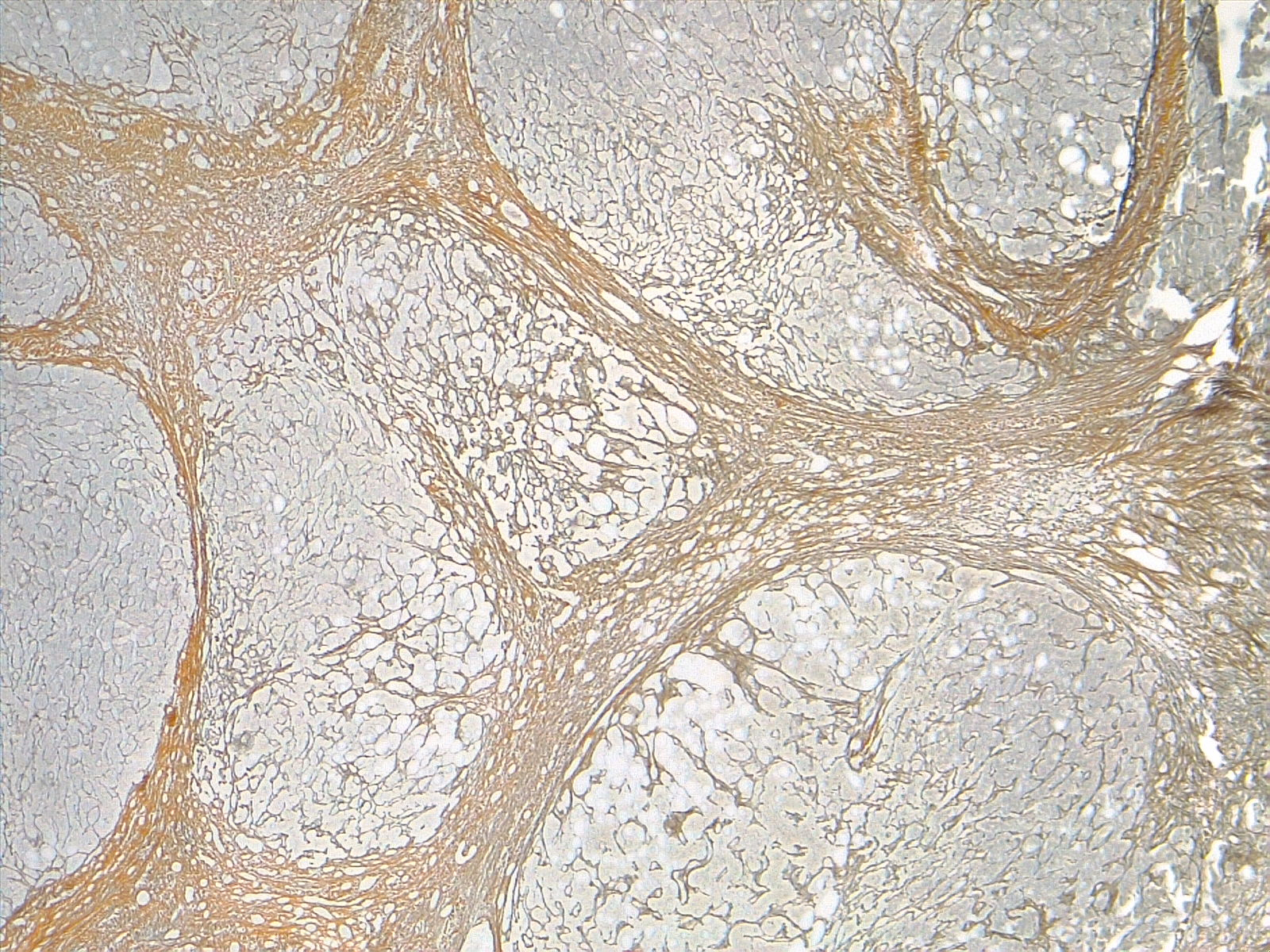
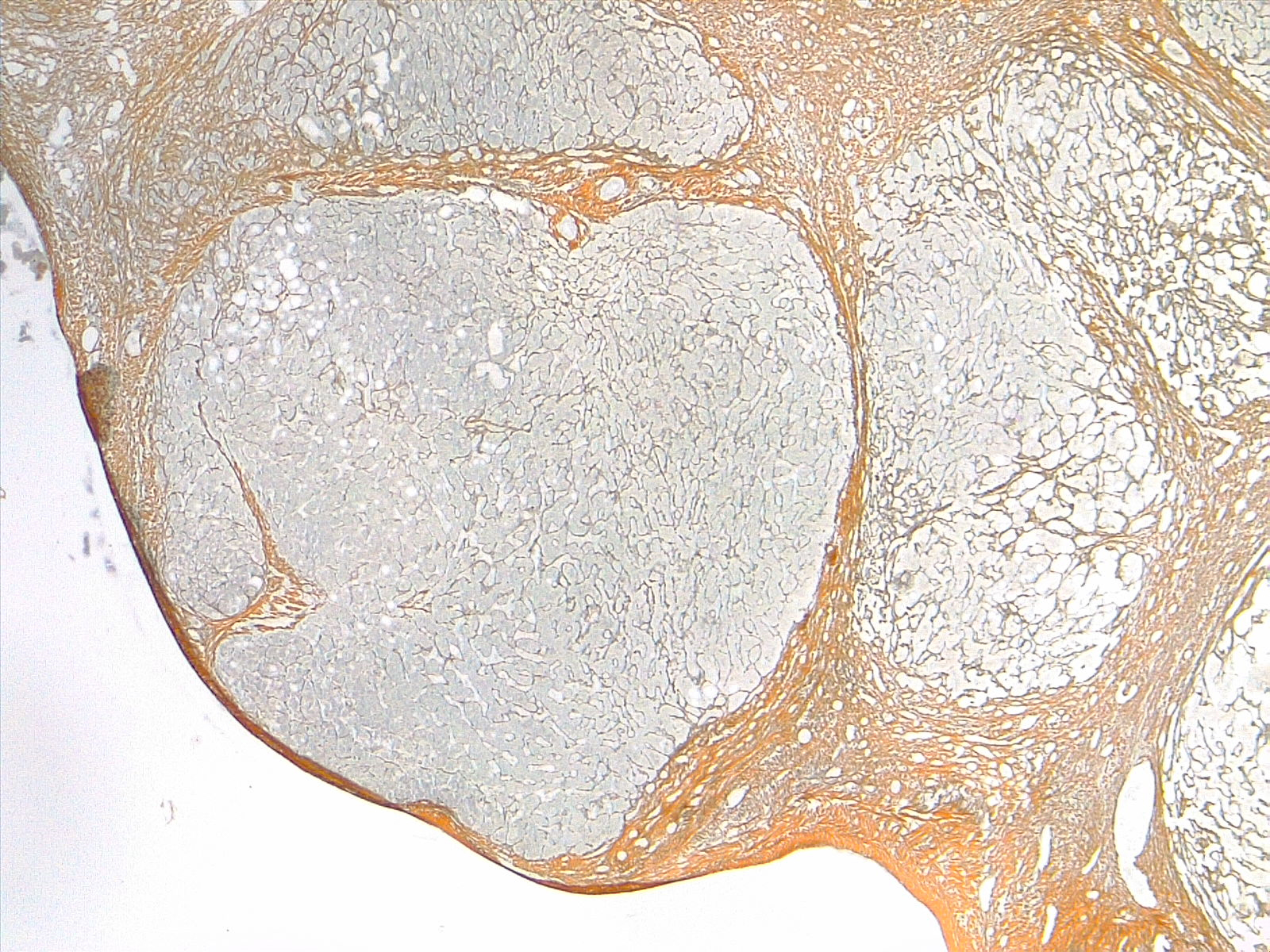
Morphologically, cirrhosis is (1) micronodular, (2) macronodular, or (3) mixed. This classification is not as clinically useful as etiologic classification. Micronodular cirrhosis (uniform nodules less than 3 mm in diameter): Cirrhosis due to alcohol, hemochromatosis, hepatic venous outflow obstruction, chronic biliary obstruction, jejunoileal bypass, and Indian childhood cirrhosis. Macronodular cirrhosis (irregular nodules with a variation greater than 3 mm in diameter): Cirrhosis due to hepatitis B and C, alpha-1 antitrypsin deficiency, and primary biliary cholangitis. Mixed cirrhosis (when micronodular and macronodular cirrhosis features are present): Usually, micronodular cirrhosis progresses into macronodular cirrhosis over time. See images. Liver Cirrhosis, Reticulin Stain 4×, Liver Cirrhosis, Trichrome Stain, 4×.
Etiology Classification
Based on the cause of cirrhosis which is sub-classified as follows:
- Viral - hepatitis B, C, and D
- Toxins - alcohol, drugs
- Autoimmune - autoimmune hepatitis
- Cholestatic - primary biliary cholangitis, primary sclerosing cholangitis
- Vascular - Budd-Chiari syndrome, sinusoidal obstruction syndrome, cardiac cirrhosis
Metabolic - hemochromatosis, NASH, Wilson disease, alpha-1 antitrypsin deficiency, cryptogenic cirrhosis.
History and Physical
Patients with cirrhosis can be asymptomatic or symptomatic, depending on whether their cirrhosis is clinically compensated or decompensated. In compensated cirrhosis, patients are usually asymptomatic, and their disease is detected incidentally by labs, physical exams, or imaging. One of the common findings is mild to moderate elevation in aminotransferases or gamma-glutamyl transpeptidase with possible enlarged liver or spleen on the exam. On the other hand, patients with decompensated cirrhosis usually present with a wide range of signs and symptoms arising from a combination of liver dysfunction and portal hypertension. The diagnosis of ascites, jaundice, hepatic encephalopathy, variceal bleeding, or hepatocellular carcinoma in a patient with cirrhosis signifies the transition from a compensated to a decompensated phase of cirrhosis. Other cirrhosis complications include spontaneous bacterial peritonitis and hepatorenal syndrome, which occur in patients who have ascites.
Multiple Organs Affected
Gastrointestinal
Portal hypertension can cause ascites, hepatosplenomegaly, and prominence of the periumbilical abdominal veins, resulting in caput medusa. Esophageal varices are another complication of cirrhosis secondary to increased blood flow in the collateral circulation, with a mortality rate of at least 20% at 6 weeks after a bleeding episode.[10] Patients with alcoholic cirrhosis are at increased risk of small bowel bacterial overgrowth and chronic pancreatitis, and patients with chronic liver disease have a higher rate of gallstone formation.[11][12]
Hematologic
Anemia can occur due to folate deficiency, hemolytic anemia (spur cell anemia in severe alcoholic liver disease), and hypersplenism. There can be pancytopenia due to hypersplenism in portal hypertension, impaired coagulation, disseminated intravascular coagulation, and hemosiderosis in cirrhosis patients due to different causes.
Renal
Patients with cirrhosis are prone to develop hepatorenal syndrome secondary to systemic hypotension and renal vasoconstriction, causing the underfilling phenomenon. Splanchnic vasodilation in cirrhosis leads to decreased effective blood flow to the kidneys, activating the renin-angiotensin-aldosterone system, leading to sodium and water retention and renal vascular constriction.[13] However, this effect is not enough to overcome the systemic vasodilation caused by cirrhosis, leading to renal hypoperfusion and worsened by renal vasoconstriction with the endpoint of renal failure.[14]
Pulmonary
Manifestations of cirrhosis include hepatopulmonary syndrome, portopulmonary hypertension, hepatic hydrothorax, decreased oxygen saturation, ventilation-perfusion mismatch, reduced pulmonary diffusion capacity, and hyperventilation.
Skin
Spider nevi, central arterioles surrounded by multiple smaller vessels resembling a spider, are seen in cirrhosis patients secondary to hyperestrogenemia. Liver dysfunction leads to a sex hormone imbalance, causing an increased estrogen-to-free testosterone ratio and the formation of spider nevi.[15] Palmar erythema is another skin finding that is seen in cirrhosis and is also secondary to hyperestrogenemia. Jaundice is a yellowish discoloration of the skin and mucous membranes seen when the serum bilirubin is greater than 3 mg/dL and in decompensated cirrhosis.
Endocrine
Patients with alcoholic liver cirrhosis can develop hypogonadism and gynecomastia. The pathophysiology is multifactorial, mainly due to the hypersensitivity of estrogen and androgen receptors seen in cirrhotic patients. Hypothalamic pituitary dysfunction has also been implicated in the development of these conditions.[16] Hypogonadism can lead to decreased libido and impotence in males with loss of secondary sexual characteristics and feminization. Women can develop amenorrhea and irregular menstrual bleeding, as well as infertility.
Nail Changes
Clubbing, hypertrophic osteoarthropathy, and the Dupuytren contracture are seen. Other nail changes include azure lunules (Wilson disease), Terry nails, and Muehrcke nails.
Others
Fetor hepaticus (sweet, musty breath smell due to high levels of dimethyl sulfide and ketones in the blood) and asterixis (flapping tremor when the arms are extended and the hands are dorsiflexed) are both features of hepatic encephalopathy that can be seen in cirrhosis.[17] Cirrhosis can lead to hyperdynamic circulation, reduced lean muscle mass, muscle cramps, and umbilical herniation. Physical examination in patients with cirrhosis may reveal stigmata of chronic liver disease (spider telangiectasias, palmar erythema, Dupuytren contractures, gynecomastia, testicular atrophy), signs of portal hypertension (ascites, splenomegaly, caput medusae, Cruveilhier-Baumgarten murmur- epigastric venous hum), signs of hepatic encephalopathy (confusion, asterixis, and fetor hepaticus), and other features such as jaundice, bilateral parotid enlargement, and scant chest/axillary hair.
Evaluation
Lab Findings
Aminotransferases are usually mildly to moderately elevated, with aspartate aminotransferase (AST) greater than alanine aminotransferase (ALT); however, normal levels do not exclude cirrhosis.[18] In most forms of chronic hepatitis (except alcoholic hepatitis), the AST/ALT ratio is less than 1. As chronic hepatitis progresses to cirrhosis, there is a reversal of this AST/ALT ratio. Alkaline phosphatase (ALP), 5'- nucleotidase, and gamma-glutamyl transferase (GGT) are elevated in cholestatic disorders. Prothrombin time (PT) is elevated due to coagulation factor defects and bilirubin, while albumin is low as the liver synthesizes it, and its functional capacity decreases. Thus, serum albumin and PT are true indicators of synthetic hepatic function. Normochromic anemia is seen; however, macrocytic anemia can be seen in alcoholic liver cirrhosis. Leukopenia and thrombocytopenia are also seen secondary to sequestration by the enlarged spleen and alcohol suppression effect on the bone marrow.[19] Immunoglobulins, especially the gamma fraction, are usually elevated due to impaired clearance by the liver.[20]
Specific Labs to Investigate Newly Diagnosed Cirrhosis
Serology and PCR techniques for viral hepatitis and autoimmune antibodies (anti-nuclear antibodies [ANA], anti-smooth muscle antibodies (ASMA), anti-liver-kidney microsomal antibodies type 1 (ALKM-1) and serum IgG immunoglobulins) for autoimmune hepatitis and anti-mitochondrial antibody for primary biliary cholangitis may be ordered. Ferritin and transferrin saturation for hemochromatosis, ceruloplasmin, and urinary copper for Wilson disease, Alpha 1-antitrypsin level, and protease inhibitor phenotype for alpha 1-antitrypsin deficiency, and serum alpha-fetoprotein for hepatocellular carcinoma (HCC) are other useful tests.
Imaging and Liver Biopsy
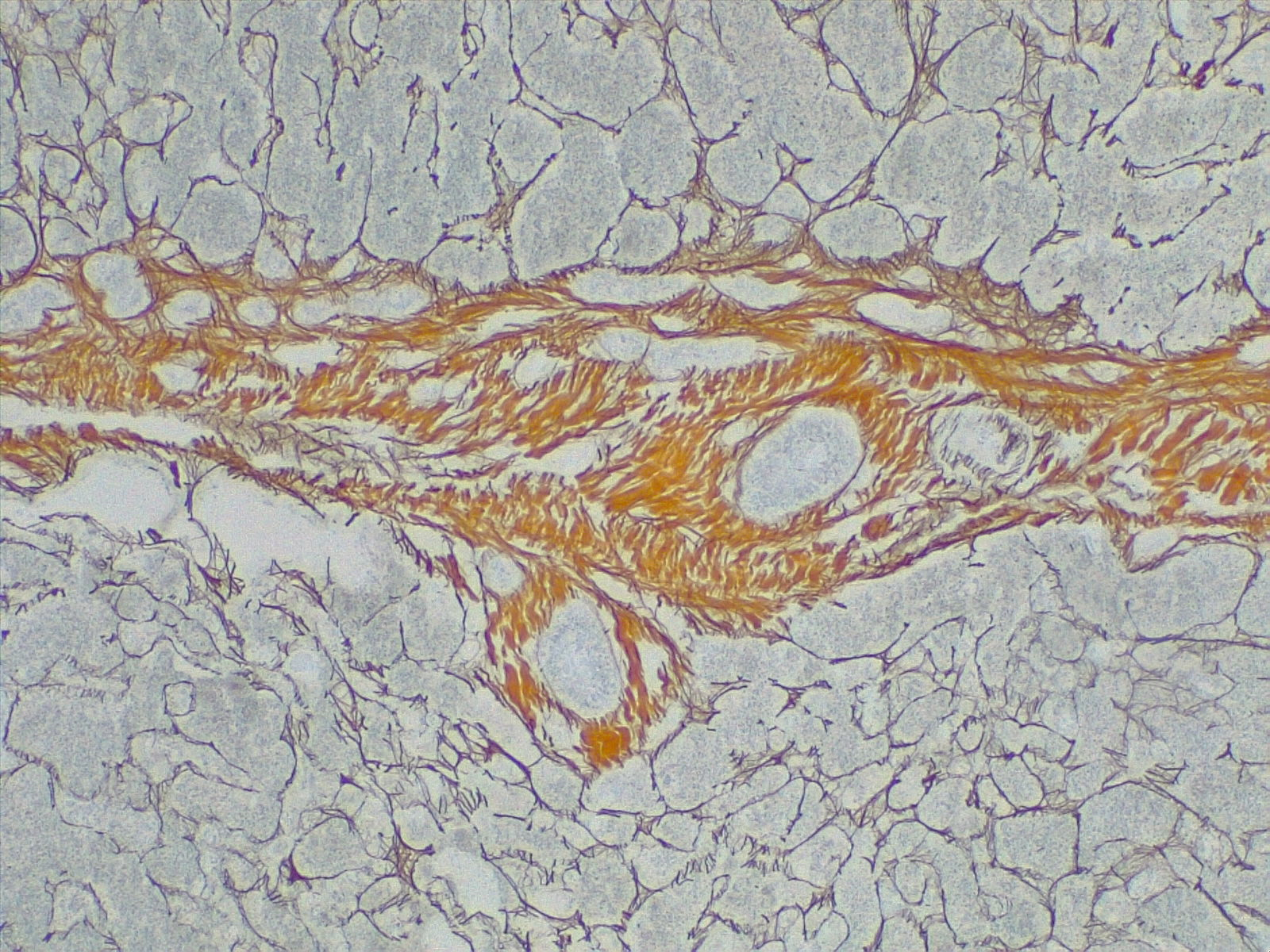
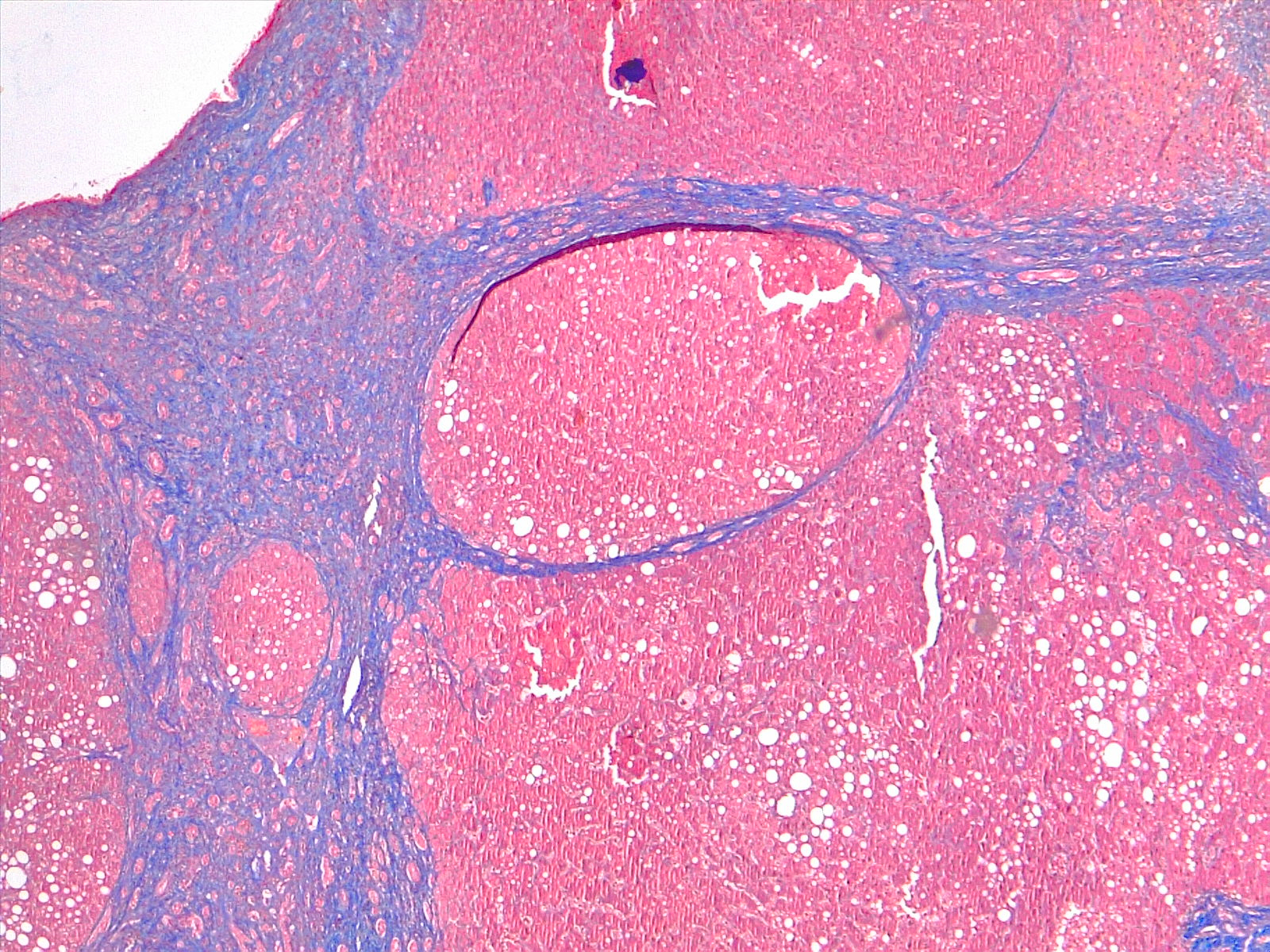
Several imaging modalities are used alongside labs to help diagnose cirrhosis. These include ultrasound, CT, MRI, and transient elastography (fibroscan). Ultrasonography is a cheap, noninvasive, and available modality for evaluating cirrhosis. It can detect nodularity and increased echogenicity of the liver, which are seen in cirrhosis; however, it is nonspecific as these findings can also be seen in fatty liver.[21] It can also determine the ratio of the caudate lobe width to the right lobe width, which usually increases in cirrhosis.[22] Moreover, it is a useful screening tool for HCC in cirrhotic patients. Duplex Doppler ultrasonography helps to assess the patency of hepatic, portal, and mesenteric veins. CT and MRI, in contrast, can detect HCC and vascular lesions, with MRI being superior to CT.[23] MRI can also be used to detect the level of iron and fat deposition in the liver for hemochromatosis, steatosis, and biliary obstruction if an MRC (magnetic resonance cholangiography) is obtained.[24] [25] MRI, however, is expensive and not readily available. Transient elastography (fibroscan) is a noninvasive method that uses high-velocity ultrasound waves to measure liver stiffness, which correlates with fibrosis. In cirrhosis, a colloid liver spleen scan using technetium-99m sulfur colloid may show increased colloid uptake in the bone marrow and spleen compared to the liver. The presence of varices in the esophagus or stomach on esophagogastroduodenoscopy (EGD) suggests portal hypertension. A liver biopsy is the gold standard for diagnosing cirrhosis and assessing the disease's degree of inflammation (grade) and fibrosis (stage). See Image. Cirrhosis, Portal Space in Fibrous Septa. Reticulin stain 4×.
Nevertheless, it can miss the diagnosis at times due to sampling errors.[24] The diagnosis of cirrhosis by biopsy requires the presence of fibrosis and nodules. The nodular pattern can be micronodular, macronodular, or mixed with the micronodular pattern, representing an independent risk factor for elevated hepatic venous pressure gradient (HVPG) and more severe disease.[24] Noninvasive tests using direct and indirect serum markers detect patients with significant fibrosis/cirrhosis from patients with no/mild fibrosis.[25][26][27]
Treatment / Management
Damage to the liver is permanent. Nevertheless, further injury to the liver should be avoided to halt the progression of the disease. General management to prevent chronic liver disease includes avoidance of alcohol, vaccination for HBV and HCV, good nutrition with a balanced diet, weight reduction, and early treatment of precipitating factors like dehydration, hypotension, and infections (see Image. Schistosomiasis Infection). This is achieved by routine monitoring of volume status, kidney function, varices development, and progression to HCC. Specific therapy usually targets the etiology, including antiviral medications in viral hepatitis, steroids and immunosuppressant agents in autoimmune hepatitis, ursodeoxycholic acid and obeticholic acid in primary biliary cholangitis, copper chelation in Wilson disease, and iron chelation and phlebotomy in hemochromatosis. Weight loss of at least 7% is beneficial in NASH, and alcohol abstinence is crucial in alcoholic cirrhosis.[28]
Differential Diagnosis
The differential diagnosis for hepatic cirrhosis includes the following:
- Acute fatty liver of pregnancy
- Amanita phalloides mushroom poisoning
- Acetaminophen poisoning
- Bacillus cereus toxin
- Fructose intolerance
- Galactosemia
- HELLP(hemolysis, elevated liver enzymes, low platelets) syndrome of pregnancy
- Hemorrhage viruses (Ebola virus, Lassa virus, Marburg virus)
- Idiopathic drug reaction
- Neonatal iron storage diseases
- Tyrosinemia
Prognosis
Predictive models for the prognosis of cirrhosis estimate the 10-year survival in patients with compensated cirrhosis at 47%, but this drops to 16% once a decompensating event occurs. The Child-Turcotte-Pugh (CTP) scoring or classification uses serum albumin, bilirubin, PT, ascites, and hepatic encephalopathy to classify patients with cirrhosis into classes A, B, and C. 1 and 2-year survival rates for these classes are 100% and 85% (A), 80% and 60% (B), and 45% and 35% (C). The model for end-stage liver disease (MELD) score is another model used to predict the short-term mortality of patients with cirrhosis. It uses serum bilirubin, creatinine, and INR to predict mortality within the next 3 months.[29] Based on the MELD score (more recently, the MELDNa score), the priority of organ allocation for liver transplantation for patients with cirrhosis is adjudicated in the US.[29] Liver transplantation is indicated in decompensated cirrhosis that does not respond to medical treatment. The 1-year and 5-year survival rates after liver transplantation are approximately 85% and 72%, respectively. Recurrence of the underlying liver disease can occur after a transplant.[30] Long-term side effects of immunosuppressant drugs are another cause of morbidity in transplant patients.
Complications
Complications accompanying hepatic cirrhosis can include:
- Portal hypertension
- Edema in the abdomen and lower extremities
- Jaundice
- Splenomegaly
- Infections
- Hemorrhage
- Hepatic encephalopathy[31]
Deterrence and Patient Education
While patient lifestyle changes cannot cure cirrhosis, these behavioral modifications can prevent or at least delay disease progression and provide symptomatic relief. Modifiable lifestyle factors include:
- Eliminating ethanol consumption
- Dietary interventions
- Avoid raw seafood and shellfish
- possible low-sodium diet to reduce water retention
- Vaccinations for pneumonia, influenza, and hepatitis
- Regulate protein intake according to their doctor's directions
- Some doctors will recommend a vitamin and mineral supplement
Pearls and Other Issues
Hepatocellular Carcinoma
HCC is the most common primary cancer in the liver, and its incidence is increasing.[32] Cirrhosis secondary to HBV and HCV is the most common risk factor.[32] Routine monitoring of cirrhotic patients for the development of HCC is recommended, with at least 6 monthly screenings using abdominal ultrasonography.[2]
Enhancing Healthcare Team Outcomes
An interprofessional team that includes a hepatologist, gastroenterologist, liver surgeon, pathologist, infectious disease specialist, primary care provider, and internist is best for treating and preventing liver cirrhosis. All healthcare workers should follow patients with liver dysfunction from any cause because it can quickly become irreversible. Liver cirrhosis is associated with many systemic complications that can cause death. A liver transplant is not always an option because of the shortage of donors.