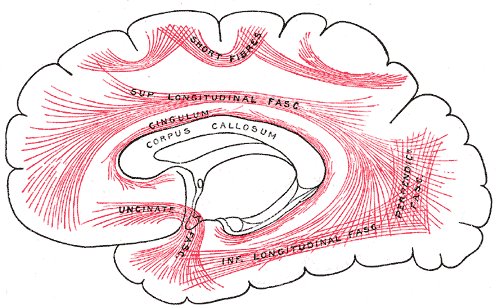
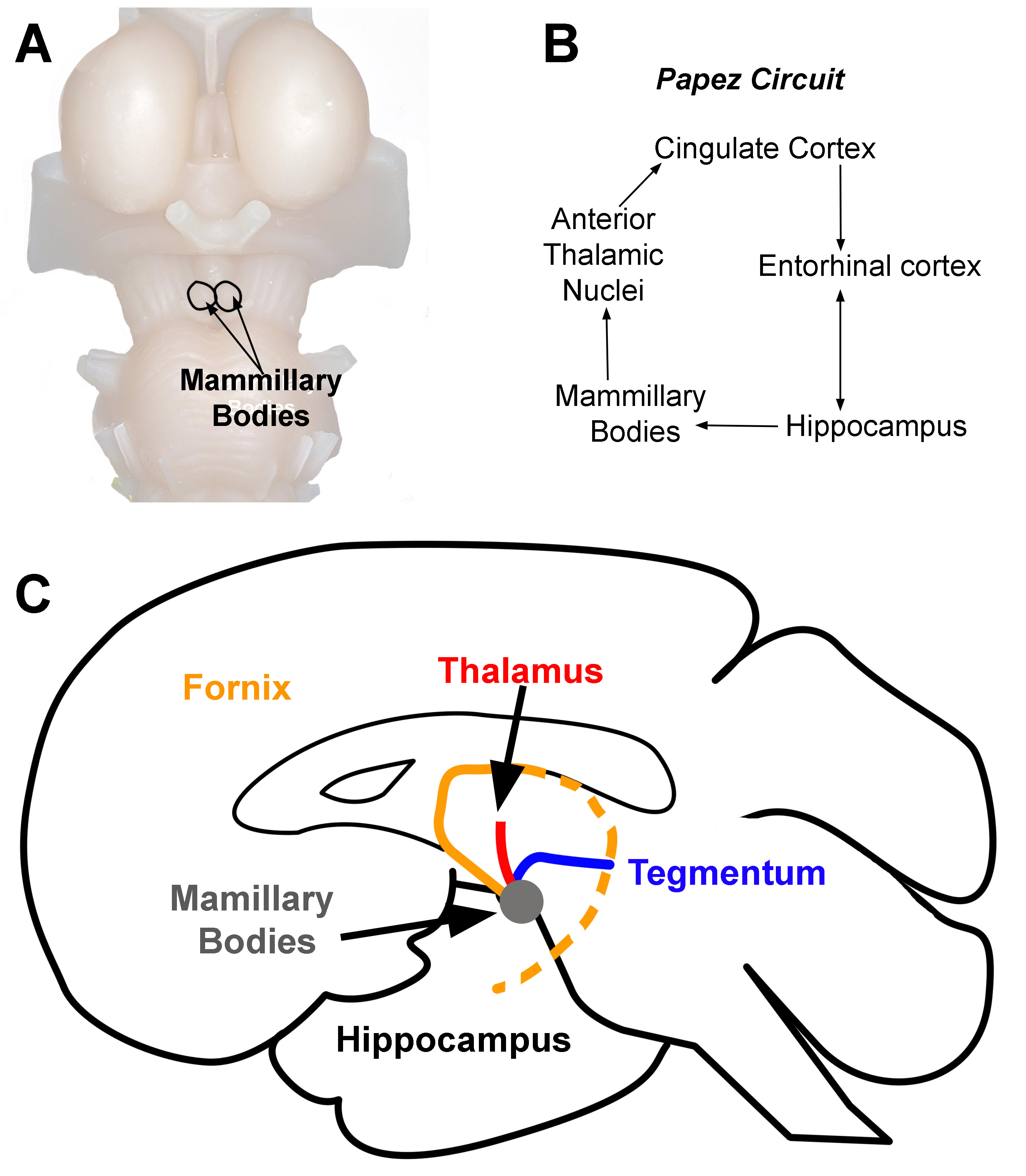
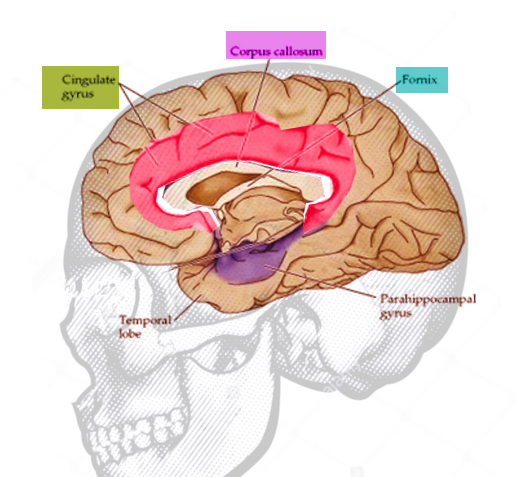
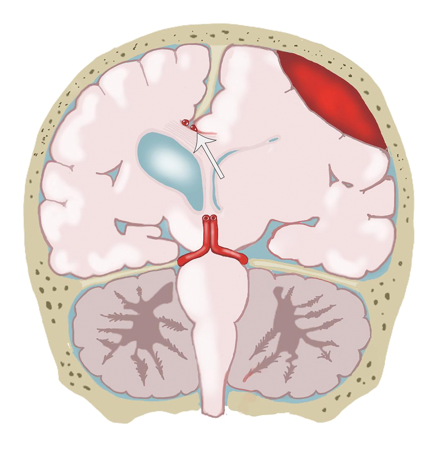
[1]
Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handbook of clinical neurology. 2019:166():23-37. doi: 10.1016/B978-0-444-64196-0.00002-9. Epub
[PubMed PMID: 31731913]
[2]
Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences. 2011 Spring:23(2):121-5
[PubMed PMID: 21677237]
[3]
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. The Journal of comparative neurology. 1995 Aug 28:359(3):490-506
[PubMed PMID: 7499543]
Level 2 (mid-level) evidence
[4]
Vogt BA, Midcingulate cortex: Structure, connections, homologies, functions and diseases. Journal of chemical neuroanatomy. 2016 Jul
[PubMed PMID: 26993424]
[5]
Strick PL, Dum RP, Picard N. Motor areas on the medial wall of the hemisphere. Novartis Foundation symposium. 1998:218():64-75; discussion 75-80, 104-8
[PubMed PMID: 9949816]
[6]
Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in brain research. 2005:150():205-17
[PubMed PMID: 16186025]
[7]
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology. 2014 Jan:137(Pt 1):12-32. doi: 10.1093/brain/awt162. Epub 2013 Jul 18
[PubMed PMID: 23869106]
[8]
Weininger J, Roman E, Tierney P, Barry D, Gallagher H, Murphy P, Levins KJ, O'Keane V, O'Hanlon E, Roddy DW. Papez's Forgotten Tract: 80 Years of Unreconciled Findings Concerning the Thalamocingulate Tract. Frontiers in neuroanatomy. 2019:13():14. doi: 10.3389/fnana.2019.00014. Epub 2019 Feb 18
[PubMed PMID: 30833890]
[9]
Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience and biobehavioral reviews. 2018 Sep:92():104-127. doi: 10.1016/j.neubiorev.2018.05.008. Epub 2018 May 16
[PubMed PMID: 29753752]
[10]
Rajmohan V, Mohandas E. The limbic system. Indian journal of psychiatry. 2007 Apr:49(2):132-9. doi: 10.4103/0019-5545.33264. Epub
[PubMed PMID: 20711399]
[11]
Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Mar 3:30(9):3339-46. doi: 10.1523/JNEUROSCI.4874-09.2010. Epub
[PubMed PMID: 20203193]
[12]
Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral cortex (New York, N.Y. : 1991). 2009 Mar:19(3):640-57. doi: 10.1093/cercor/bhn117. Epub 2008 Jul 24
[PubMed PMID: 18653667]
[13]
Cilliers K, Page BJ. Review of the Anatomy of the Distal Anterior Cerebral Artery and Its Anomalies. Turkish neurosurgery. 2016:26(5):653-61. doi: 10.5137/1019-5149.JTN.14294-15.1. Epub
[PubMed PMID: 27337235]
[14]
Pujol J, López A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage. 2002 Apr:15(4):847-55
[PubMed PMID: 11906225]
[15]
Steele JD, Christmas D, Eljamel MS, Matthews K. Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biological psychiatry. 2008 Apr 1:63(7):670-7
[PubMed PMID: 17916331]
Level 2 (mid-level) evidence
[16]
Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurgery clinics of North America. 2003 Apr:14(2):225-35
[PubMed PMID: 12856490]
[17]
Starkweather CK, Bick SK, McHugh JM, Dougherty DD, Williams ZM. Lesion location and outcome following cingulotomy for obsessive-compulsive disorder. Journal of neurosurgery. 2022 Jan 1:136(1):221-230. doi: 10.3171/2020.11.JNS202211. Epub 2021 Jul 9
[PubMed PMID: 34243154]
[18]
Brown LT,Mikell CB,Youngerman BE,Zhang Y,McKhann GM 2nd,Sheth SA, Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. Journal of neurosurgery. 2016 Jan
[PubMed PMID: 26252455]
Level 1 (high-level) evidence
[19]
Bourne SK, Sheth SA, Neal J, Strong C, Mian MK, Cosgrove GR, Eskandar EN, Dougherty DD. Beneficial effect of subsequent lesion procedures after nonresponse to initial cingulotomy for severe, treatment-refractory obsessive-compulsive disorder. Neurosurgery. 2013 Feb:72(2):196-202; discussion 202. doi: 10.1227/NEU.0b013e31827b9c7c. Epub
[PubMed PMID: 23147780]
[20]
Rück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, Nyman H, Asberg M, Svanborg P. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Archives of general psychiatry. 2008 Aug:65(8):914-21. doi: 10.1001/archpsyc.65.8.914. Epub
[PubMed PMID: 18678796]
[21]
Tayebi Meybodi A, Tabani H, Benet A. Arachnoid and dural reflections. Handbook of clinical neurology. 2020:169():17-54. doi: 10.1016/B978-0-12-804280-9.00002-0. Epub
[PubMed PMID: 32553288]
[22]
Leinonen V,Vanninen R,Rauramaa T, Raised intracranial pressure and brain edema. Handbook of clinical neurology. 2017;
[PubMed PMID: 28987174]
[23]
Riveros Gilardi B, Muñoz López JI, Hernández Villegas AC, Garay Mora JA, Rico Rodríguez OC, Chávez Appendini R, De la Mora Malváez M, Higuera Calleja JA. Types of Cerebral Herniation and Their Imaging Features. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Oct:39(6):1598-1610. doi: 10.1148/rg.2019190018. Epub
[PubMed PMID: 31589570]
[24]
Johnson PL, Eckard DA, Chason DP, Brecheisen MA, Batnitzky S. Imaging of acquired cerebral herniations. Neuroimaging clinics of North America. 2002 May:12(2):217-28
[PubMed PMID: 12391633]
[25]
Byard RW. Patterns of cerebral and cerebellar herniation. Forensic science, medicine, and pathology. 2013 Jun:9(2):260-4. doi: 10.1007/s12024-012-9339-9. Epub 2012 Apr 29
[PubMed PMID: 22544455]
[26]
Wang L,Hosakere M,Trein JC,Miller A,Ratnanather JT,Barch DM,Thompson PA,Qiu A,Gado MH,Miller MI,Csernansky JG, Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophrenia research. 2007 Jul
[PubMed PMID: 17433626]
Level 2 (mid-level) evidence
[27]
Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophrenia research. 2007 Jul:93(1-3):1-12
[PubMed PMID: 17399954]
Level 1 (high-level) evidence
[28]
Benes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Archives of general psychiatry. 1987 Jul:44(7):608-16
[PubMed PMID: 3606326]