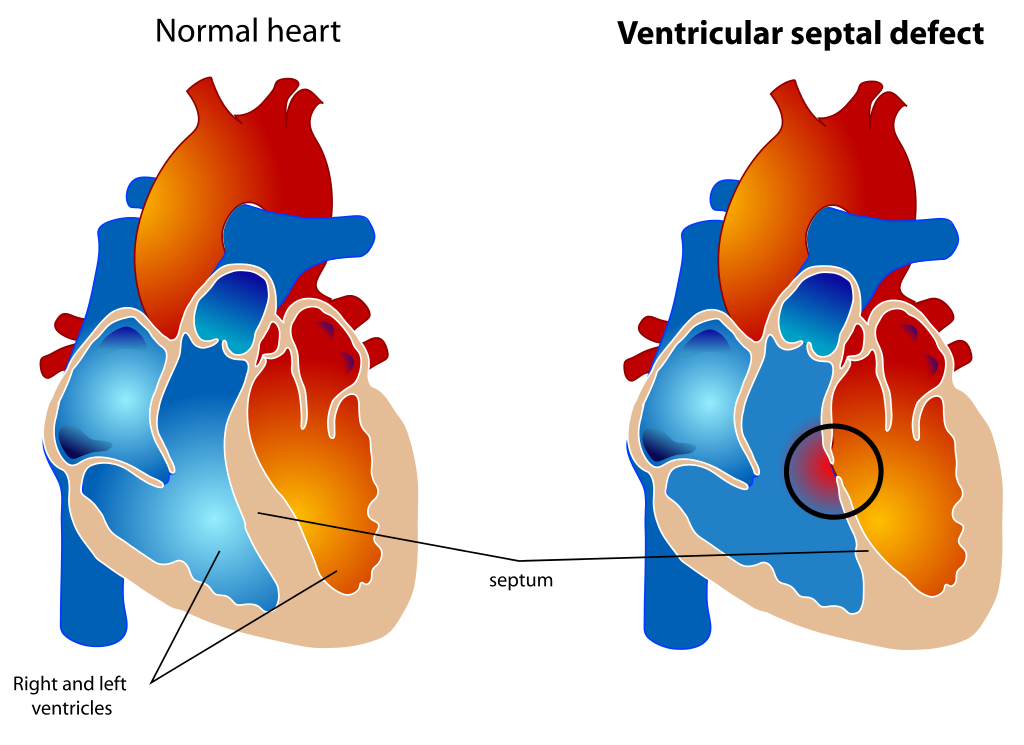
[1]
Liu F, Yang YN, Xie X, Li XM, Ma X, Fu ZY, Chen BD, Huang Y, Shan CF, Ma YT, Gao XM. Prevalence of Congenital Heart Disease in Xinjiang Multi-Ethnic Region of China. PloS one. 2015:10(8):e0133961. doi: 10.1371/journal.pone.0133961. Epub 2015 Aug 28
[PubMed PMID: 26317413]
[2]
Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation. 1988 Aug:78(2):361-8
[PubMed PMID: 3396173]
[3]
Lin CH, Huddleston C, Balzer DT. Transcatheter ventricular septal defect (VSD) creation for restrictive VSD in double-outlet right ventricle. Pediatric cardiology. 2013 Mar:34(3):743-7. doi: 10.1007/s00246-012-0337-1. Epub 2012 May 12
[PubMed PMID: 22580772]
[4]
Kutty S, Delaney JW, Latson LA, Danford DA. Can we talk? Reflections on effective communication between imager and interventionalist in congenital heart disease. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013 Aug:26(8):813-27. doi: 10.1016/j.echo.2013.05.006. Epub 2013 Jun 13
[PubMed PMID: 23768692]
[5]
Thakkar B, Patel N, Bohora S, Bhalodiya D, Singh T, Madan T, Shah S, Poptani V, Shukla A. Transcatheter device closure of perimembranous ventricular septal defect in children treated with prophylactic oral steroids: acute and mid-term results of a single-centre, prospective, observational study. Cardiology in the young. 2016 Apr:26(4):669-76. doi: 10.1017/S1047951115001018. Epub 2015 Jun 24
[PubMed PMID: 26105182]
Level 2 (mid-level) evidence
[6]
Jortveit J, Leirgul E, Eskedal L, Greve G, Fomina T, Døhlen G, Tell GS, Birkeland S, Øyen N, Holmstrøm H. Mortality and complications in 3495 children with isolated ventricular septal defects. Archives of disease in childhood. 2016 Sep:101(9):808-13. doi: 10.1136/archdischild-2015-310154. Epub 2016 Apr 18
[PubMed PMID: 27091847]
[7]
Xie YM, Zhang ZW, Li YF, Qian MY, Wang HS. [Management of the arrhythmia around the procedure of transcatheter closure of ventricular septal defects in pediatric patients]. Zhonghua xin xue guan bing za zhi. 2005 Dec:33(12):1092-4
[PubMed PMID: 16563277]
Level 2 (mid-level) evidence
[8]
Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K, Investigators of the European VSD Registry. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. European heart journal. 2007 Oct:28(19):2361-8
[PubMed PMID: 17684082]
[9]
Chessa M, Carrozza M, Butera G, Negura D, Piazza L, Giamberti A, Feslova V, Bossone E, Vigna C, Carminati M. The impact of interventional cardiology for the management of adults with congenital heart defects. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2006 Feb:67(2):258-64
[PubMed PMID: 16416475]
[10]
Matyal R, Wang A, Mahmood F. Percutaneous ventricular septal defect closure with Amplatzer devices resulting in severe tricuspid regurgitation. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013 Nov 15:82(6):E817-20. doi: 10.1002/ccd.24803. Epub 2013 Jul 1
[PubMed PMID: 23553968]
[11]
Durham JA, Scansen BA, Bonagura JD, Schober KE, Cheatham SL, Cheatham JP. Iatrogenic embolization and transcatheter retrieval of a ventricular septal defect occluder in a dog. Journal of veterinary cardiology : the official journal of the European Society of Veterinary Cardiology. 2015 Dec:17(4):304-13. doi: 10.1016/j.jvc.2015.08.003. Epub 2015 Oct 26
[PubMed PMID: 26515420]
[12]
Nguyen HL, Phan QT, Dinh LH, Tran HB, Won H, Thottian JJ, Duc DD, Quang TN, Kim SW. Nit-Occlud Lê VSD coil versus Duct Occluders for percutaneous perimembranous ventricular septal defect closure. Congenital heart disease. 2018 Jul:13(4):584-593. doi: 10.1111/chd.12613. Epub 2018 Jul 17
[PubMed PMID: 30019378]
[13]
Bambul Heck P, Eicken A, Kasnar-Samprec J, Ewert P, Hager A. Early pulmonary arterial hypertension immediately after closure of a ventricular or complete atrioventricular septal defect beyond 6months of age. International journal of cardiology. 2017 Feb 1:228():313-318. doi: 10.1016/j.ijcard.2016.11.056. Epub 2016 Nov 9
[PubMed PMID: 27866021]