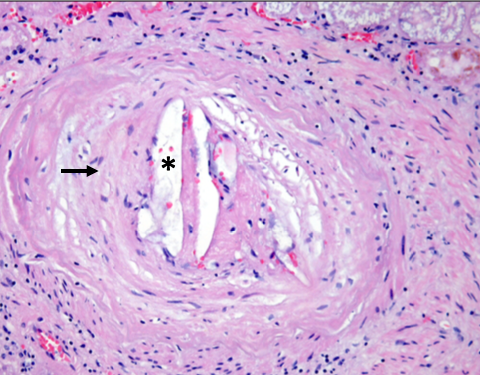
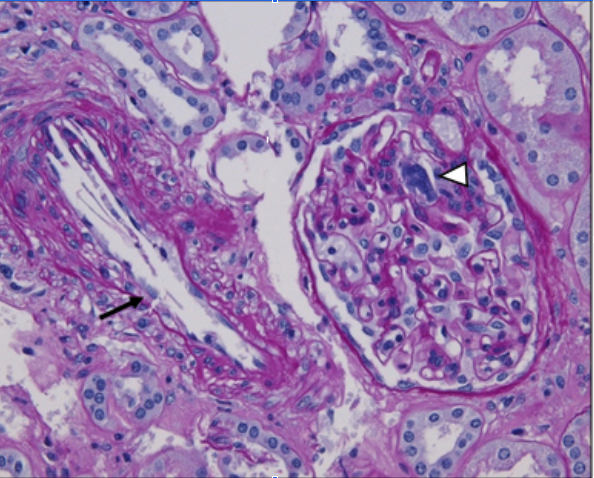
[1]
Carvajal JA, Anderson WR, Weiss L, Grismer J, Berman R. Atheroembolism. An etiologic factor in renal insufficiency, gastrointestinal hemorrhages, and peripheral vascular diseases. Archives of internal medicine. 1967 Jun:119(6):593-9
[PubMed PMID: 5298059]
[2]
Darsee JR. Cholesterol embolism: the great masquerader. Southern medical journal. 1979 Feb:72(2):174-80
[PubMed PMID: 371003]
[3]
Cappiello RA, Espinoza LR, Adelman H, Aguilar J, Vasey FB, Germain BF. Cholesterol embolism: a pseudovasculitic syndrome. Seminars in arthritis and rheumatism. 1989 May:18(4):240-6
[PubMed PMID: 2727705]
[4]
Ștefan G, Zugravu A, Stancu S, Gherghiceanu M, Terinte-Balcan G. Atheroembolic kidney disease: The under-recognized silent killer. Clinical case reports. 2021 Mar:9(3):1824-1825. doi: 10.1002/ccr3.3874. Epub 2021 Jan 28
[PubMed PMID: 33768956]
Level 3 (low-level) evidence
[5]
Chaudhary S, Kashani KB. Acute Kidney Injury Management Strategies Peri-Cardiovascular Interventions. Interventional cardiology clinics. 2023 Oct:12(4):555-572. doi: 10.1016/j.iccl.2023.06.008. Epub 2023 Aug 5
[PubMed PMID: 37673499]
[6]
Li X, Bayliss G, Zhuang S. Cholesterol Crystal Embolism and Chronic Kidney Disease. International journal of molecular sciences. 2017 May 24:18(6):. doi: 10.3390/ijms18061120. Epub 2017 May 24
[PubMed PMID: 28538699]
[7]
Vassalotti JA, Delgado FA, Whelton A. Atheroembolic Renal Disease. American journal of therapeutics. 1996 Jul:3(7):544-549
[PubMed PMID: 11862288]
[8]
Scolari F, Ravani P. Atheroembolic renal disease. Lancet (London, England). 2010 May 8:375(9726):1650-60. doi: 10.1016/S0140-6736(09)62073-0. Epub 2010 Apr 8
[PubMed PMID: 20381857]
[9]
Mittal BV, Alexander MP, Rennke HG, Singh AK. Atheroembolic renal disease: a silent masquerader. Kidney international. 2008 Jan:73(1):126-30
[PubMed PMID: 17667989]
[10]
Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010 Aug 10:122(6):631-41. doi: 10.1161/CIRCULATIONAHA.109.886465. Epub
[PubMed PMID: 20697039]
[11]
Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A, Cholesterol Embolism Study(CHEST) Investigators. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. Journal of the American College of Cardiology. 2003 Jul 16:42(2):211-6
[PubMed PMID: 12875753]
[12]
Modi KS, Rao VK. Atheroembolic renal disease. Journal of the American Society of Nephrology : JASN. 2001 Aug:12(8):1781-1787. doi: 10.1681/ASN.V1281781. Epub
[PubMed PMID: 11461954]
[13]
Scott T, Ethier I, Hawley C, Pascoe EM, Viecelli AK, Ng A, Cho Y, Johnson DW. Burden of kidney failure from atheroembolic disease and association with survival in people receiving dialysis in Australia and New Zealand: a multi-centre registry study. BMC nephrology. 2021 Dec 2:22(1):401. doi: 10.1186/s12882-021-02604-7. Epub 2021 Dec 2
[PubMed PMID: 34856938]
[14]
Pirson Y, Honhon B, Cosyns JP, van Ypersele C. Cholesterol embolism in a renal graft after treatment with streptokinase. British medical journal (Clinical research ed.). 1988 Feb 6:296(6619):394-5
[PubMed PMID: 3125916]
[15]
Singh I, Killen PD, Leichtman AB. Cholesterol emboli presenting as acute allograft dysfunction after renal transplantation. Journal of the American Society of Nephrology : JASN. 1995 Aug:6(2):165-70
[PubMed PMID: 7579080]
[16]
Ripple MG, Charney D, Nadasdy T. Cholesterol embolization in renal allografts. Transplantation. 2000 May 27:69(10):2221-5
[PubMed PMID: 10852632]
[17]
Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Nephrotoxic risks of renal angiography: contrast media-associated nephrotoxicity and atheroembolism--a critical review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1994 Oct:24(4):713-27
[PubMed PMID: 7942832]
[18]
Yamamoto N, Sakai N, Kaikoi D, Yomogida D, Kajikawa S, Wada T, Yuasa T, Ogura H, Sato K, Miyagawa T, Kitajima S, Toyama T, Hara A, Shimizu M, Wada T, Iwata Y. Cholesterol Crystal Embolism: Autopsy-proven Gastrointestinal Lesions, Pancreatitis, and End-stage Kidney Disease Which Developed after Undergoing Selective Abdominal Angiography. Internal medicine (Tokyo, Japan). 2024 May 9:():. doi: 10.2169/internalmedicine.3469-24. Epub 2024 May 9
[PubMed PMID: 38719599]
[19]
Scolari F, Ravani P, Gaggi R, Santostefano M, Rollino C, Stabellini N, Colla L, Viola BF, Maiorca P, Venturelli C, Bonardelli S, Faggiano P, Barrett BJ. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007 Jul 17:116(3):298-304
[PubMed PMID: 17606842]
[20]
Scolari F, Tardanico R, Zani R, Pola A, Viola BF, Movilli E, Maiorca R. Cholesterol crystal embolism: A recognizable cause of renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000 Dec:36(6):1089-109
[PubMed PMID: 11096032]
[21]
Polu KR, Wolf M. Clinical problem-solving. Needle in a haystack. The New England journal of medicine. 2006 Jan 5:354(1):68-73
[PubMed PMID: 16394304]
[22]
Piranavan P, Rajan A, Jindal V, Verma A. A rare presentation of spontaneous atheroembolic renal disease: A case report. World journal of nephrology. 2019 Jun 28:8(3):67-74. doi: 10.5527/wjn.v8.i3.67. Epub 2019 Jun 10
[PubMed PMID: 31363463]
Level 3 (low-level) evidence
[23]
Mulay SR, Anders H-J. Crystallopathies. The New England journal of medicine. 2016 Sep 29:375(13):e29. doi: 10.1056/NEJMc1609332. Epub
[PubMed PMID: 27682056]
[24]
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Mar 9:440(7081):237-41
[PubMed PMID: 16407889]
[25]
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010 Apr 29:464(7293):1357-61. doi: 10.1038/nature08938. Epub
[PubMed PMID: 20428172]
[26]
Corr EM, Cunningham CC, Dunne A. Cholesterol crystals activate Syk and PI3 kinase in human macrophages and dendritic cells. Atherosclerosis. 2016 Aug:251():197-205. doi: 10.1016/j.atherosclerosis.2016.06.035. Epub 2016 Jun 22
[PubMed PMID: 27356299]
[27]
Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S. Human Mincle Binds to Cholesterol Crystals and Triggers Innate Immune Responses. The Journal of biological chemistry. 2015 Oct 16:290(42):25322-32. doi: 10.1074/jbc.M115.645234. Epub 2015 Aug 20
[PubMed PMID: 26296894]
[28]
Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegård KT, Brekke OL, Lambris JD, Damås JK, Latz E, Mollnes TE, Espevik T. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. Journal of immunology (Baltimore, Md. : 1950). 2014 Mar 15:192(6):2837-45. doi: 10.4049/jimmunol.1302484. Epub 2014 Feb 19
[PubMed PMID: 24554772]
[29]
Niyonzima N, Samstad EO, Aune MH, Ryan L, Bakke SS, Rokstad AM, Wright SD, Damås JK, Mollnes TE, Latz E, Espevik T. Reconstituted High-Density Lipoprotein Attenuates Cholesterol Crystal-Induced Inflammatory Responses by Reducing Complement Activation. Journal of immunology (Baltimore, Md. : 1950). 2015 Jul 1:195(1):257-64. doi: 10.4049/jimmunol.1403044. Epub 2015 May 29
[PubMed PMID: 26026058]
[30]
Nymo S, Niyonzima N, Espevik T, Mollnes TE. Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology. 2014 Oct:219(10):786-92. doi: 10.1016/j.imbio.2014.06.006. Epub 2014 Jul 5
[PubMed PMID: 25053140]
[31]
Scoble JE, O'Donnell PJ. Renal atheroembolic disease: the Cinderella of nephrology? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996 Aug:11(8):1516-7
[PubMed PMID: 8856200]
[32]
Nasri H, Mubarak M. Contrast induced nephropathy has to be differentiated from kidney injury due to atheroembolic disease. Journal of renal injury prevention. 2013:2(3):107-8. doi: 10.12861/jrip.2013.34. Epub 2013 Sep 1
[PubMed PMID: 25340143]
[33]
Sakan H, Nakatani K, Asai O, Matsui M, Iwano M, Saito Y. [Case of purpura nephritis accompanied by idiopathic cholesterol embolism]. Nihon Jinzo Gakkai shi. 2012:54(5):622-8
[PubMed PMID: 22991843]
Level 3 (low-level) evidence
[34]
Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Seminars in dialysis. 2010 Nov-Dec:23(6):606-13. doi: 10.1111/j.1525-139X.2010.00769.x. Epub 2010 Dec 20
[PubMed PMID: 21166875]
[35]
Yang L, Steiger S, Shi C, Gudermann T, Mammadova-Bach E, Braun A, Anders HJ. Both hyperglycemia and hyperuricemia aggravate acute kidney injury during cholesterol embolism syndrome despite opposite effects on kidney infarct size. Kidney international. 2023 Jul:104(1):139-150. doi: 10.1016/j.kint.2023.03.016. Epub 2023 Mar 30
[PubMed PMID: 37001603]
[36]
Desai M, Ram R, Prayaga A, Dakshinamurty KV. Cholesterol crystal embolization (CCE): Improvement of renal function with high-dose corticosteroid treatment. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2011 Mar:22(2):327-30
[PubMed PMID: 21422636]
[38]
Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. The American journal of cardiology. 2011 Jun 15:107(12):1710-7. doi: 10.1016/j.amjcard.2011.02.336. Epub 2011 Apr 18
[PubMed PMID: 21507364]
[39]
Thériault J, Agharazzi M, Dumont M, Pichette V, Ouimet D, Leblanc M. Atheroembolic renal failure requiring dialysis: potential for renal recovery? A review of 43 cases. Nephron. Clinical practice. 2003:94(1):c11-8
[PubMed PMID: 12806187]
Level 3 (low-level) evidence
[40]
Scolari F, Ravani P, Pola A, Guerini S, Zubani R, Movilli E, Savoldi S, Malberti F, Maiorca R. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. Journal of the American Society of Nephrology : JASN. 2003 Jun:14(6):1584-90
[PubMed PMID: 12761259]
[41]
Faria B, Vidinha J, Pêgo C, Garrido J, Lemos S, Lima C, Sorbo G, Gomes EL, Carvalho T, Loureiro P, Sousa T. Atheroembolic renal disease with rapid progression and fatal outcome. Clinical and experimental nephrology. 2011 Feb:15(1):159-63. doi: 10.1007/s10157-010-0363-3. Epub 2010 Nov 11
[PubMed PMID: 21069411]
[42]
Frank RD. [Cholesterol embolism syndrome: a rare, but severe complication in patients with atherosclerosis]. Deutsche medizinische Wochenschrift (1946). 2012 May:137(21):1130-4. doi: 10.1055/s-0032-1305005. Epub 2012 May 15
[PubMed PMID: 22588660]