Continuing Education Activity
This activity reviews the evaluation and management of antepartum infections and highlights the role of the interprofessional team in improving maternal and fetal outcomes in these conditions.
Objectives:
- Identify the etiology of antepartum infections and their impact on maternal and fetal morbidity and mortality.
- Describe the appropriate evaluation of antepartum infections.
- Outline the management options available for antepartum infections.
- Discuss inter-professional team strategies for improving care coordination and communication to advance the care of antepartum infections and improve outcomes.
Introduction
Antepartum infections are a significant contributor to maternal and fetal morbidity and mortality. Populations in countries with developing economies bear the brunt of the burden of these infections.[1][2] However, a growing distrust of the medical establishment and vaccination skepticism in wealthier countries has resulted in a resurgence of many of these preventable infections.[3][4] Understanding these infections and their manifestations is critical not only for diagnosis and management but also for the provision of anticipatory care and guidance to pregnant patients and their families. The following is a discussion of vertically-transmitted antepartum infections, infections that affect the maternal uterus and birth canal, and infections that disproportionately affect pregnant women. While this review only discusses those classes of infections in detail, it is vital to note that any infection present in the general population can present in a pregnant patient and consequently impact the pregnancy.
Etiology
Pregnancy results in a state of immune alteration, which is needed to prevent the destruction of the fetus by the maternal immune system.[5] Infections acquired during pregnancy can be passed vertically to the developing fetus through the placenta or to the infant through birth or breastfeeding. The ToRCHS organisms are a group of congenital infections acquired through vertical transmission. ToRCHS stands for toxoplasmosis, rubella, cytomegalovirus, human immunodeficiency virus (HIV), and syphilis. Other significant antepartum infections include herpes simplex virus (HSV), hepatotropic viruses (mainly HBV and HCV), parvovirus B19, and Zika virus, which will be discussed in conjunction with the ToRCHS infections. Hepatitis E (HEV) disproportionately affects pregnant women, and there is evidence that it may be vertically transmitted as well.[6]
Infections of the female urogenital tract can similarly impact birth outcomes, even though they are not always vertically-transmitted to the newborn. Such infections include bacterial vaginosis, mostly by group B streptococci (GBS). Bacterial vaginosis is an abnormal overgrowth of bacteria occurring in the setting of pH changes. GBS colonizes genitourinary tracts in many women but can cause significant fetal morbidity and mortality if left untreated; the most significant for prevalence is chorioamnionitis occurring by ascending infection. Chorioamnionitis or intraamniotic infection (IAI) is a condition wherein the fetal chorion and amnion become inflamed and infected.
Epidemiology
Vertically transmitted infections are disproportionately prevalent in countries with developing economies.[7] For example, 11% of European women of childbearing age are seropositive for toxoplasmosis compared to 77% of an age-matched population in South America.[7] Areas of a notably high prevalence of infection include high rates of syphilis in Central Africa, cytomegalovirus (CMV) in South America, hepatitis B in west sub-Saharan Africa, hepatitis C in the Middle East and eastern Asia, hepatitis E in India, Southeast Asia, the Middle East, and Africa, and HIV in southern Africa.[7] Zika virus is of particular importance as an emerging infectious disease notable for its effect on fetal development. Primary infections have been identified in Africa, Asia, South America, and the Pacific islands.[8]
Infections of the female urogenital tract are common worldwide. Bacterial vaginosis is estimated to affect nearly one-third of women of childbearing age in the United States, although approximately 80% of those affected were asymptomatic.[9] The incidence of chorioamnionitis is inversely related to gestational age at delivery, with neonates delivered in the peri-viable period having a 94% chance of chorioamnionitis.[10] Maternal factors that increase the risk for chorioamnionitis include nulliparity, immune-compromised states, bacterial vaginosis, GBS or Ureaplasma urealyticum colonization, tobacco use, alcohol or drug abuse, and African American ethnicity.[10] Labor-related factors associated with increased risk include preterm or prolonged premature rupture of membranes, use of internal monitoring during labor, repeat vaginal examinations, and epidural anesthesia.[10] Finally, between 15 and 35% of women of childbearing age in the United States are colonized with GBS in the vagina or rectum.[11]
Pathophysiology
The hormonally-mediated physiologic changes of pregnancy increase maternal risk for certain infections and may likewise increase the severity of disease in these patients.[5] Vertical transmission to the fetus can occur through transplacental migration of the organism prenatally, contact with maternal blood and vaginal secretions during labor, or exposure to infected breast milk after birth.[7] The mechanism of initial maternal infection varies with the etiologic agent.
Toxoplasma gondii causes toxoplasmosis, a protozoan that can cause human infection through ingestion of raw or undercooked meat or contaminated product, contact with cat feces or contaminated food or soil, or transfusion of infected blood products or donor organs.[7] Vertical transmission of toxoplasmosis is through transplacental infection.[7] The risk of congenital infection increases linearly with gestational age at the time of primary maternal infection.[7]
Maternal rubella infection occurs following the exposure of a nonimmune individual to aerosolized viral particles.[7] Transmission to the fetus is transplacental, and the highest risk of transmission is with maternal infection in the first trimester.[7]
Maternal HIV infection occurs through contact with infected bodily fluids, including through sexual contact, needle sticks, or blood transfusions.[7] Vertical transmission is most common in utero but can also occur intrapartum and through breastfeeding.[7] High maternal viral plasma load, low maternal CD4 count, and acute maternal infection during pregnancy increase the risk of in utero transmission.[7]
Primary CMV is acquired through contact with infected bodily fluids, including saliva, urine, cervical or vaginal secretions, semen, blood, and breastmilk.[12] The risk of vertical transmission is estimated to be approximately 35% and can occur transplacentally, during birth, or through exposure to infected breast milk.[12] Infection early in gestation is less common but associated with more severe manifestations.[12]
The Treponema pallidum causes syphilis. Maternal infection occurs through sexual contact, and congenital infection is transmitted transplacentally with increased risk in mothers with high spirochete titers, an early stage of infection at the time of pregnancy, and late or incomplete treatment.[7] Primary HSV infection occurs through sexual contact; vertical transmission occurs primarily through contact with active lesions on the maternal birth canal during parturition.[13] Less common modes of transmission include transplacental infection and postnatal infection.[13]
Primary infection with parvovirus B19 occurs through exposure to respiratory droplets or infected blood products. Congenital infection is transplacental.[7]
Primary infection with HBV is through contact with infected bodily fluids, with sexual contact being the most common cause of primary infection in the United States.[14] Vertical transmission accounts for approximately a third of HBV infections worldwide and results primarily from contact with contaminated bodily fluids during labor and delivery.[7] Transplacental infection accounts for a minority of congenital HBV infections.[7] Primary HCV is spread through contact with contaminated body fluids, and the mechanism of its vertical transmission has not been delineated.[7] Primary infection with HEV occurs through contaminated food and water or contact with infected blood. It is notable for its severity in pregnant women, with high mortality during the third trimester.[15] In endemic areas, it is a major contributor to maternal death, fulminant hepatic failure, and fetal loss.[16]
The mosquitoes spread the Zika virus in the Aedes genus, and an unknown primate reservoir is theorized to exist.[8] There have been anecdotal of suspected primary infection through sexual contact, but data are lacking to support this as a mechanism.[8] Vertical transmission occurs in utero.[17]
The majority of cases of chorioamnionitis occur secondary to ascending infection with maternal urogenital organisms in the setting of ruptured fetal membranes. As such, the most commonly implicated organisms in chorioamnionitis are the aerobic and anaerobic bacteria, which typically colonize the urinary and genital tracts, including Ureaplasma urealyticum, Mycoplasma hominis, GBS, Escherichia coli, and Gardnerella vaginalis.[10] It is uncertain if the association between ascending urogenital infection and ruptured membranes is secondary to opportunistic infection by these organisms when the anatomic barrier or the fetal membranes is removed or if infection of the membranes results in the weakening of the fetal membranes and leads to premature rupture.[10]
A less common cause of chorioamnionitis is the hematogenous transplacental spread of infection from mother to fetus.[10] This mechanism has been classically described in chorioamnionitis caused by Listeria monocytogenes, which causes chorioamnionitis in the setting of intact fetal membranes.[18] More rare mechanisms of chorioamnionitis include direct iatrogenic inoculation of the amniotic fluid during invasive procedures such as amniocentesis or chorionic villous sampling and spread of peritoneal infection to the fetal membranes through the fallopian tubes.[10] Patients with chronic liver and kidney disease are at particular risk for the spread of infection from the peritoneum through the fallopian tubes.[10]
Bacterial vaginosis occurs secondary to an imbalance of the physiologic vaginal flora, in which Lactobacillus sp. usually are predominant.[19] It is theorized that this imbalance starts with the formation of biofilms by Gardnerella vaginalis, which then allows for the opportunistic growth of other aerobic and anaerobic species.[20] The association between preterm birth and bacterial vaginosis is suspected to be secondary to an increased risk of chorioamnionitis and, therefore, preterm birth in patients with bacterial vaginosis.[21] The weakening of the fetal membranes by these bacteria leading to premature rupture may also contribute.[21]
Finally, maternal colonization with GBS is an essential contributor to neonatal morbidity and mortality. Neonatal infection can occur through ascending vaginal infection or through contact during parturition.[22]
Histopathology
The pathology report in a placenta specimen examines the gross and microscopic findings, significatively helping providers involved in patient's care. The report has three main functions: provide information that fills the gap in the clinicopathological events that resulted in complications during pregnancy, provides information that may help for immediate therapy such as unrecognized antepartum infection, and gives information that may be precious for a future pregnancy. The placental pathology report includes a gross and microscopic description with a final diagnosis. The provider can review the pathological diagnosis with the pathologist if needed.
It is of paramount importance the recognition of the source of the inflammatory response: it can be either maternal or fetal. When the inflammatory response is fetal, there are associations with neurological impairment in the offspring. Fetal inflammation of the umbilical cord is a strong marker of fetal infection.[23] In different clinical studies, chorioamnionitis is strongly related to low weight at birth and cerebral palsy, thus suggesting relevant causal links with prematurity and inflammation.[24] Severe fetal inflammation with neutrophils and eosinophils is predictive of an adverse neonatal outcome, especially when preterm. In a Canadian registry-based cohort study[25] of 1229 selected patients, a strong link between neonatal infection and sensorineural auditory impairment was found. The current pathogenic hypothesis that premyelinating oligodendrocytes are vulnerable to inflammation mediated by cytokines.[26]
The location and characteristics of inflammation should be documented. The topography is essential to determine if the inflammatory response is maternal or fetal. Chorioamnionitis, deciduitis, and inflammation of the chorionic plate is a maternal inflammatory response. In contrast, choriositis and inflammation of the umbilical cord and the chorionic vessels (on the chorionic plate of the placenta) is of fetal origin. As previously said, there is evidence that fetal sequelae are associated with fetal inflammation.[27][28] Chronic inflammation implies different forms of response and should be documented.
The gross examination may reveal the opacification of the membranes (loss of the shiny surface of the amnion), and the color may change from transparent to off-white, yellowish, or greenish. The site of membrane rupture is the most important to check microscopically because it is where the inflammation process is usually more prominent, as well as around the site of insertion into the placental plate. White spots can suggest Candida spp. infection.
There are specific grading and staging systems for the maternal and fetal inflammatory response (Amsterdam[29] and Redline[23] criteria). Staging refers to the anatomical progression of the process; grading refers to the intensity of the acute inflammation.
- Stage 1: the presence of neutrophils in the chorion or subchorionic space; 2: the presence of neutrophils in the chorionic connective tissue or amnion; 3: necrotizing chorioamnionitis and epithelial necrosis of the amnion
- Grade 1: small clusters of maternal neutrophils, found in chorion laeve, chorionic plate, subchorionic fibrin, amnion; 2: three or more confluent groups of 10-20 neutrophils (microabscesses)
Subchorial Inflammation: It is the earliest form of inflammation and is defined by the presence of neutrophils between the decidua and chorion levae; it does not necessarily imply the presence of chorioamnionitis. In the early phases, there is a patchy accumulation of leukocytes, accumulation of fibrin at the choriodecidual interface in the decidua. Extension of the infiltrate into the chorionic layer is called chorionitis and can be followed by the involvement of the amniotic layer (chorioamnionitis).
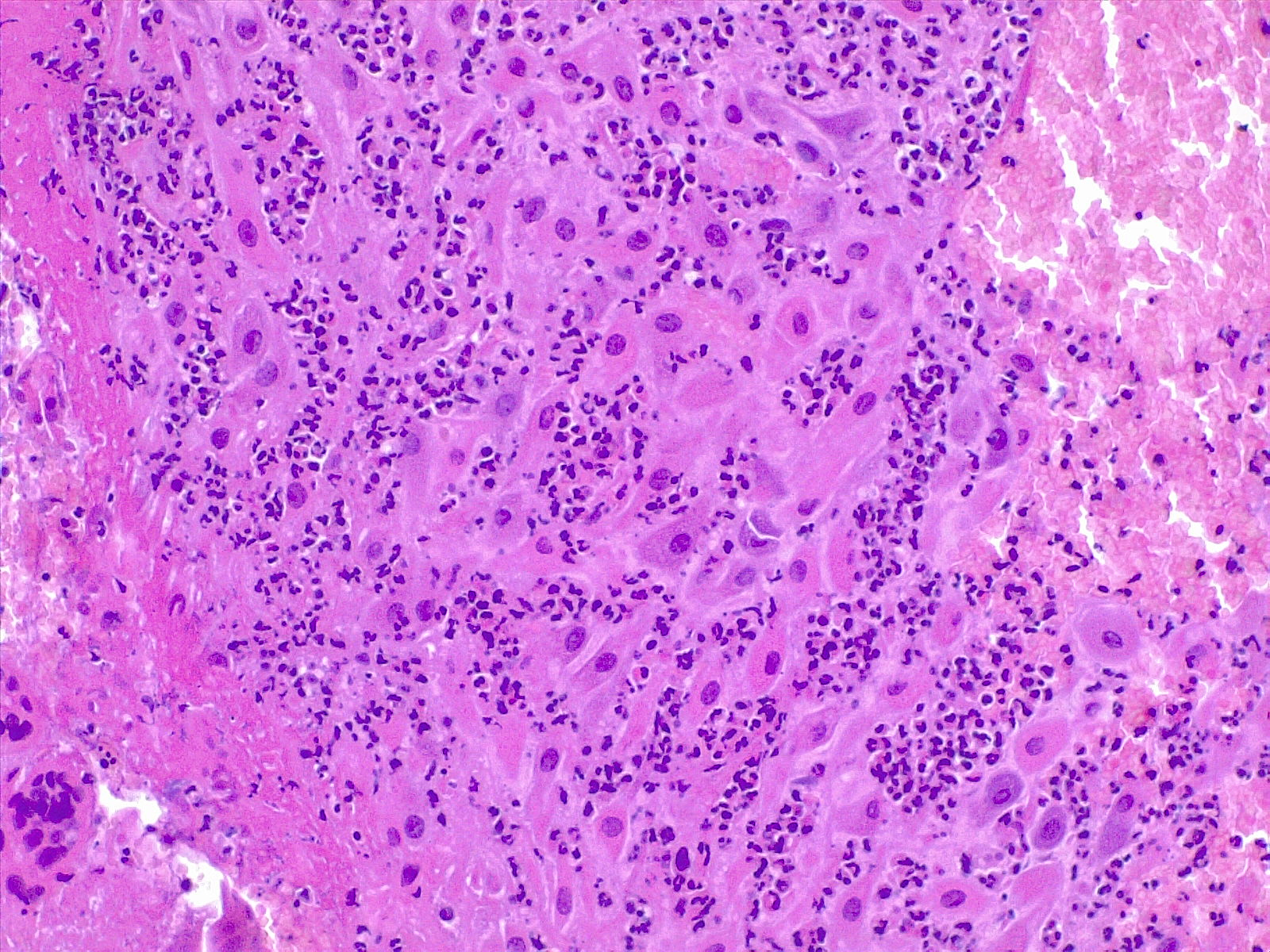
Chorioamnionitis refers to the presence of inflammation in the chorion and amnion, and it is the result of ascending microbial infection from the lower genital tract to the sterile amniotic cavity potentially endangering the fetus.[30] Neutrophils are generally not present in chorion or amnios and migrate from the decidua in cases of ascending or blood-borne infection. It is diagnosed by microscopic examination of placental sections, with the typical findings of neutrophilic (acute) inflammation. In acute chorioamnionitis, the leukocytes are of maternal origin. Usually, it is not necessary to use immunohistochemistry to diagnose acute chorioamnionitis. It can be useful to confirm herpes simplex or varicella infection when the suspicion is strong.
Acute Villitis: It is a specific gross and histologic feature of acute inflammation of the placental villi. It is diagnosed histologically with the presence of neutrophilic abscesses in the placental parenchyma, often associated with neutrophilic chorioamnionitis. Acute villitis is a typical feature of a bacterial infection, such as listeria or streptococcal infection.
Funisitis is the inflammatory response occurring in the umbilical cord. Acute funisitis, along with acute chorionic vasculitis, represent the fetal inflammatory response syndrome. Topographically it can present with neutrophils into the umbilical vein (or acute phlebitis), the umbilical arteries (or acute arteritis), or infiltration into the Wharton jelly. Acute funisitis usually begins with inflammation of the umbilical vein (phlebitis), followed by arteritis. The latter is evidence of an advanced inflammatory response.
Microbial colonization of the amniotic cavity can result in fetal invasion by the respiratory tract, gastrointestinal tract, the skin, the ear through the auditory canal to the middle ear. The fetal examination can reveal severe dermatitis, pneumonitis, and encephalitis. The progression of the microbial agent to fetal circulation could lead to systemic disease. The rate of congenital neonatal sepsis is higher in preterm neonates than in term neonates, probably due to bacterial infection following a preterm rupture of membranes.[31]
History and Physical
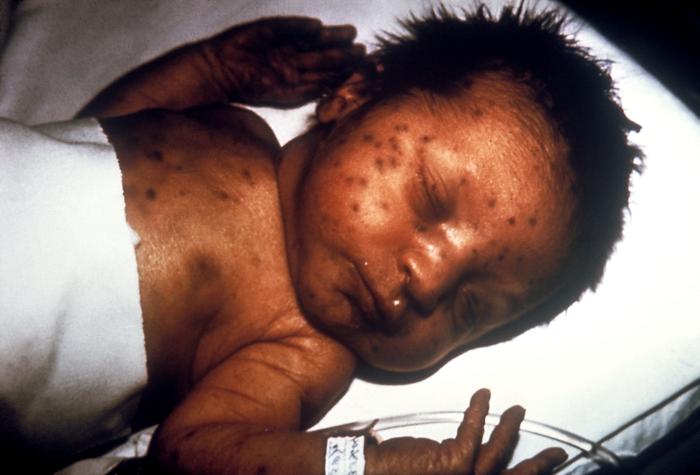
In discussing the history and physical exam findings of antepartum infections, it is important to delineate between the findings in primary (in this case, maternal) infection and congenital infection. It is likewise important to note that the manifestations of congenital infection may not be present immediately following birth. Several findings are common to many congenital infections. These include hepatomegaly, splenomegaly, thrombocytopenia, and a violaceous macular or papular rash, commonly called a "blueberry muffin rash" that results from extramedullary hematopoiesis.[7]
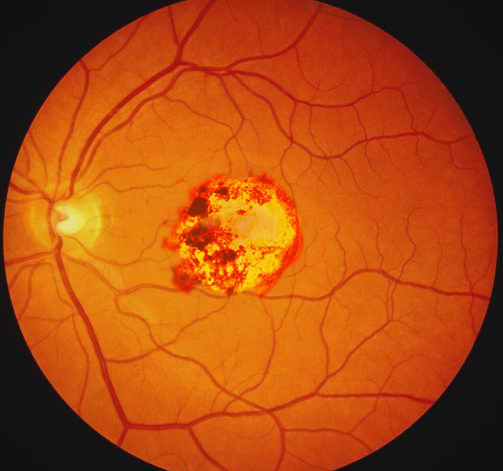
Maternal primary toxoplasmosis is asymptomatic; approximately 70 to 90% of congenitally-infected infants are likewise asymptomatic at birth.[7] Chorioretinitis, hydrocephalus, and periventricular calcifications are the classic, but rare, the triad of symptoms in congenital toxoplasmosis.[7] More common early manifestations of congenital toxoplasmosis include anemia and thrombocytopenia, hepatosplenomegaly, seizures, and jaundice.[7] Late manifestations in untreated disease include hearing loss, microcephaly, seizures, motor, and cerebellar dysfunction, and intellectual disability.[7] Primary infection with rubella in adulthood is often asymptomatic but can manifest with low-grade fevers, sore throat, conjunctivitis, and a macular rash starting on the face and spreading down.[32]
Congenital rubella syndrome (CRS) can include deafness, cardiac defects, and a variety of ophthalmic and ocular findings, including microphthalmia, cataracts, and glaucoma.[7] Primary infection with CMV is usually asymptomatic, making the diagnosis in pregnant women difficult. When present, symptoms can include a prolonged febrile illness resembling mononucleosis, sore throat, rash, and transaminitis.[12] Cerebral manifestations of congenital CMV infection include periventricular calcifications, microcephaly, parenchymal echogenic foci, cerebral pseudocysts, hypoplastic corpus callosum, and abnormal gyri.[12] These findings are often visible before delivery on fetal ultrasound.[12]
Other manifestations include ascites, hepatosplenomegaly, hydrops, pericardial effusion, and placental enlargement.[12] Primary infection with HIV is likewise usually asymptomatic, although acute infection can present with a flu-like illness. Similarly, congenital infection is usually asymptomatic in the acute phase. Failure to thrive, oral candidiasis, developmental delay, and frequent opportunistic infections are common presenting complaints.[33] Non-congenital syphilis infection has three stages: primary, secondary, and tertiary. The primary stage is characterized by the development of a painless genital or rectal chancre.[34] A rash involving the palms and soles is the most characteristic symptoms of secondary syphilis and can be macular, papular, ulcerating, or pustular.[34] Other symptoms of secondary syphilis are non-specific and can include constitutional symptoms, gastrointestinal ulcerations, musculoskeletal inflammation, and ocular complaints.[34]
Tertiary syphilis can manifest with cardiovascular complications such as aortitis and neurological abnormalities like tabes dorsalis or paresis.[35] Early manifestations of congenital syphilis include hepatosplenomegaly, nasal secretions (called "snuffles"), osteochondritis, pseudoparalysis, rash, anemia, and thrombocytopenia.[7] Untreated congenital syphilis progresses to involve the central nervous and musculoskeletal systems, resulting in developmental abnormalities as well as abnormalities of the bones, joints, teeth, eyes, and skin.[7] Active herpes infection is characterized by grouped, painful vesicular lesions. Congenital HSV can present with low birth weight, microcephaly, chorioretinitis, and hydrocephalus.[36] The characteristic vesicular lesions may also be present in neonatal infection, the feared manifestation of HSV in neonates is viral encephalitis, which can result in significant and permanent blindness, neurodevelopmental delay, and death.[37]
Maternal infection with parvovirus can be asymptomatic or may present with a distinctive "slapped cheeks" rash preceded by a flu-like illness of fever, malaise, arthralgias, and myalgias.[7] Manifestations of congenital infection include nonimmune hydrops fetalis secondary to high-output heart failure and viral myocarditis, severe anemia and thrombocytopenia, and maternal mirror syndrome.[7] Acute manifestations of primary HBV include subclinical (anicteric) or icteric hepatitis, as well as fulminant hepatitis and cirrhosis.[7] Symptoms of congenital HBV are usually absent at birth. A minority of affected neonates develop clinical hepatitis in the first few months of life and can present with jaundice, failure to thrive, and vomiting.[7] More commonly, congenital HBV results in subclinical hepatitis and an immune-tolerant carrier state, and progression to chronic infection is uncommon.[7]
Acute primary HCV infection presents similarly to HBV above. Most cases of congenital infection are asymptomatic, but, in contrast to congenital HBV infection, the majority of cases of congenital HCV result in chronic infection.[7] Primary HEV infection usually results in mild, subclinical hepatitis in the general population. Still, it has a high risk of progression to fulminant hepatitis and death in pregnant women, particularly in the third trimester.[16]
Vertical transmission has been documented but is rare.[38] Primary infection with the Zika virus can be asymptomatic or present with a "dengue-like" syndrome of nonpurulent conjunctivitis, arthralgias, headache, maculopapular rash, extremity edema, and gastrointestinal upset.[8] Congenital infection is characterized by severe microcephaly and neurological abnormalities.[39]
While definitionally a pathologic diagnosis, the diagnosis of chorioamnionitis is usually made based on clinical findings. Unfortunately, the symptoms of chorioamnionitis are non-specific and poorly sensitive for the diagnosis; as such, attention to the complete clinical picture is critical to making this diagnosis.[10] Findings suggestive of chorioamnionitis include maternal fever, uterine tenderness, maternal or fetal tachycardia, and purulent or malodorous discharge.[10] Bacterial vaginosis is usually asymptomatic; some may complain of a "fishy-smelling" discharge or vaginal itching.[40] Maternal GBS colonization is asymptomatic but increases the risk of neonatal sepsis.[41]
Evaluation
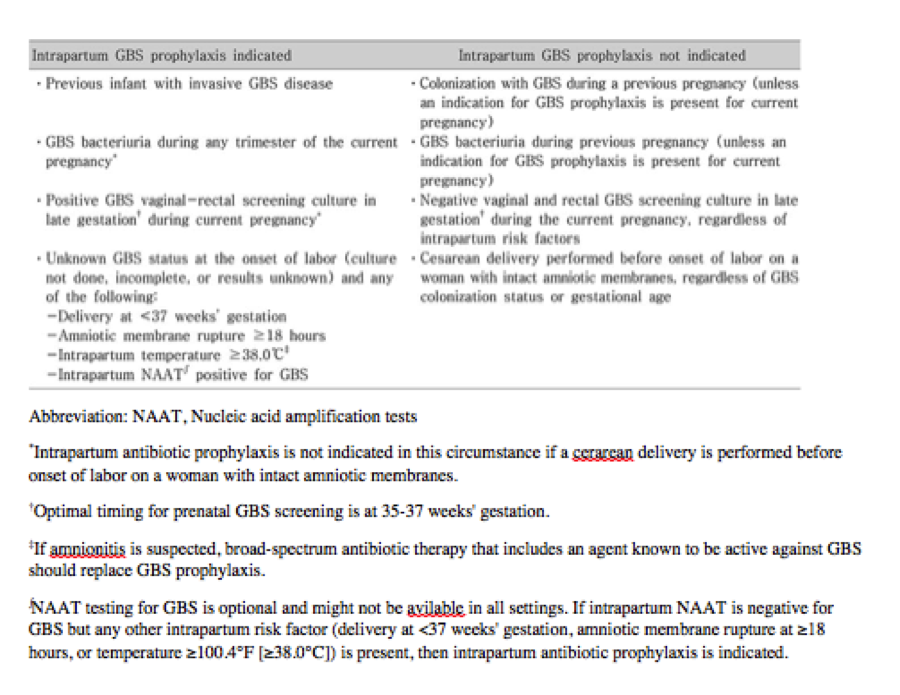
Current guidelines by the American College of Obstetricians and Gynecologists (ACOG) recommend universal screening for hepatitis B, syphilis, and chlamydia at the first prenatal visit, which should occur at 8-10 weeks of gestation.[42] Universal recommendations also include an HIV screen at the first visit using an "opt-out" approach, where testing is done unless the patient declines it.[42] All pregnant women should have a rectal and vaginal culture for GBS at 35-37 weeks of gestation; patients at high-risk for preterm labor should be tested before 35 weeks.[43]
Additional recommended screening for antepartum infections includes repeat testing for HIV and syphilis in the third trimester in populations at high-risk for the acquisition of these infections.[42] Screening for toxoplasmosis should be done for patients who have a high risk of exposure to contaminated food or water or cat litter.[44]
Hepatitis C screening should be done for patients with a history of intravenous drug use or blood transfusion or organ transplant before 1992.[45] CMV screening is recommended for patients with frequent child contact, such as daycare workers, pediatricians, and teachers.[44] Adolescents with a history of prior sexually transmitted infections or multiple partners should also be screened for CMV. HSV screening is recommended for women with a previous history of STIs and those who are immunocompromised.[7]
Infants born to HIV positive mothers or mothers with unknown HIV status should be tested for the disease using nucleic acid tests (NATs).[7] The recommended schedule includes testing within 48 hours of birth, at two weeks of life, between 4 and 6 weeks of life, and between 4 and 6 months of life.[7]
Chorioamnionitis is a pathologic diagnosis made by examination of the fetal membranes for a neutrophilic infiltrate.[10] However, this diagnosis is usually made on a clinical basis, and treatment should not be delayed for the postpartum pathologic examination of the placenta. Maternal leukocytosis supports the diagnosis of chorioamnionitis but is neither sensitive nor specific.[10] Infants exposed to chorioamnionitis should have blood cultures, a complete blood count, and a C-reactive protein (CRP) drawn at birth to evaluate for neonatal sepsis, for which they are at increased risk.[10]
The United States Preventative Services Task Force (USPSTF) recommends against routine screening for bacterial vaginosis in asymptomatic pregnant women, not at risk of preterm labor.[46] Even in those with risk factors for preterm labor, there is insufficient evidence to support a recommendation for routine screening in pregnant individuals.[46] In symptomatic patients, testing is through microscopic evaluation of vaginal discharge obtained through a vaginal swab.[19] The presence of stippled squamous epithelial cells (called "clue cells") is a diagnostic of bacterial vaginosis.[19]
Treatment / Management
In toxoplasma seropositive pregnant women diagnosed before 18 weeks gestation, treatment should begin with spiramycin until ultrasonography and PCR can evaluate for the presence of congenital fetal infection.[7] If fetal infection is present, spiramycin should be continued, and pyrimethamine sulfadiazine and folinic acid should be added to the regimen.[7] Observational data suggests decreased fetal infection and less serious neurologic sequelae with these regimens, although there is no trial data on the topic.[7]
There is no specific therapy for rubella in primary or congenital infection. The cornerstone of management is, therefore, prevention. All children and adolescents without contraindications to the MMR vaccine should receive it. MMR administration is contraindicated in pregnancy. Pregnant women found to be non-immune to rubella should be counseled on the importance of vaccination and vaccinated upon completion of the pregnancy.[47] Women of childbearing age who receive the MMR vaccine should be cautioned to avoid becoming pregnant within 28 days of vaccine administration.[7]
The treatment of CMV infection in immunocompetent pregnant women is supportive. Ganciclovir and valganciclovir are used in the treatment of congenital CMV infection. They are associated with a reduction in hearing loss and improved weight gain and head circumference in congenital CMV.[48]
Antiretroviral prophylaxis should be given to all infants born to HIV-positive mothers.[7] A 4 or 6-week course of zidovudine can be used for infant prophylaxis in cases where the mother was adherent with prepartum cART and had effective viral suppression throughout the pregnancy.[7] In cases where prenatal cART was not used, infants should receive a 6-week course of zidovudine as well as three total doses of nevirapine within 8 hours after birth, at 48 hours following the first dose, and 96 hours following the second dose.[7] The cART should be immediately initiated in HIV-positive infants. Early cART initiation is associated with reduced mortality and improved developmental milestones and gross motor skills in these infants.[7] Vaginal delivery can be considered if the risk of transmission to the fetus is low, as evidenced by low maternal viral loads; otherwise, cesarean section is recommended to reduce the risk of intrapartum HIV-transmission to the infant.[49] HIV-positive mothers should not breastfeed.[49]
The only treatment of syphilis in pregnant women is intramuscular penicillin.[7] Penicillin-allergic patients should be desensitized before treatment. Treatment for congenital syphilis is 10-days of parenteral penicillin.[50]
A primary HSV outbreak in pregnancy should be treated with acyclovir, famciclovir, or valacyclovir.[51] Oral therapy is typically sufficient, but parenteral treatment may be needed in severe or disseminated cases. The suppressive therapy should be offered at 36 weeks to women with recurrent genital herpes.[51] Known HSV infection is not a contraindication to vaginal delivery. Cesarean section should only be performed if genital lesions are present at the time of delivery.[51] Infants suspected or confirmed to have congenital HSV should receive intravenous acyclovir.[52]
Treatment of acute and chronic HBV in pregnant women is primarily supportive. Infants born to HBV-positive mothers should receive a single-antigen HBV vaccine within 12 hours of birth combined with HBV immunoglobulin.[7] Families should be counseled on the importance of completing the vaccine series for the infant. Although direct antiviral agents for HCV are now available, therapy in pregnant women has not been studied, and treatment to prevent vertical transmission is not currently recommended.[7] Treatment of HCV should be deferred in children until at least three years of age when possible to allow for potential spontaneous resolution and avoid the side effects of the treatment medications in the infant.[53] Hepatitis E treatment, both in primary infection in pregnant women and congenital cases, is supportive.[54]
Treatment of primary parvovirus infection in pregnant women is supportive. Treatment of congenital infection is likewise primarily supportive, and in utero fetal transfusions have become a cornerstone of therapy in the management of the associated severe fetal anemia.[7]
Treatment of both primary and congenital Zika virus infection is supportive, so prevention is vital. Several vaccines are in development.
Patients with preterm rupture of membranes should receive antibiotics targeting the most common organisms implicated in chorioamnionitis.[10] In the preterm rupture of membranes, antenatal antibiotics are associated with a decreased risk of neonatal sepsis, respiratory distress syndrome, and necrotizing enterocolitis.[10] In these patients, antibiotic use is also associated with increasing time for delivery.[10]
Recommended antibiotic regimens include ampicillin and gentamicin.[10] If signs of chorioamnionitis develop, urgent delivery is indicated regardless of gestational age. Neonates exposed to chorioamnionitis should be started on empiric antibiotics pending results of the blood cultures and laboratory testing described above. The duration of antibiotic use in these neonates has not been delineated. However, experts suggest that, in the setting of reassuring laboratory results and negative blood cultures, antibiotics can be discontinued after 48 hours.[10]
Diagnosed bacterial vaginosis should be treated in all pregnant women.[55] The recommended treatment is a 7-day course of metronidazole or clindamycin.[55]
Pregnant patients colonized with GBS should receive intrapartum antibiotics, with penicillin G being the medication of choice in non-allergic patients.[43] Cefazolin can be used in patients having a non-life-threatening penicillin allergy. In contrast, clindamycin should be used in patients with a history of urticaria, angioedema, respiratory distress, or anaphylaxis with penicillin administration.[43]
Differential Diagnosis
The high maternal temperature has a broad differential diagnosis that, in addition to antepartum infections, includes infections common to the general population. Following differential diagnoses should be considered in patients with prenatal infection:
- Pneumonia
- Cellulitis
- Urinary tract infections
- Pulmonary embolism
- Hyperthermic toxidromes
Genetic disorders should be considered in the differential of neonates born with suspected stigmata of congenital infections. Genetic counseling and testing should be advised to these families.
Prognosis
With the notable exception of HEV, the antepartum infections discussed above have favorable maternal prognosis in immunocompetent individuals. Maternal sequelae from toxoplasmosis, rubella, CMV, parvovirus, and Zika virus infection are rare.[7] Progression of acute hepatitis B and C to chronic infection is possible and increases the risk for hepatic failure and hepatocellular carcinoma in these patients.[7][56]
The risk of fulminant liver failure and mortality in pregnant patients with HEV ranges from 30-100%.[6] Finally, with appropriate cART, individuals with HIV-infection have a comparable life expectancy to their non-infected counterparts.[57] The prognosis of vertically-transmitted infections in the neonate depends mainly on the severity of the manifestations.
Complications
The most severe fetal complication of congenital infection is fetal demise and spontaneous abortion, but the incidence of this is difficult to estimate. Congenital toxoplasmosis, rubella, CMV, and HSV can result in low birth weight, failure to thrive, and significant neurodevelopmental and motor delays in affected infants.[7] HSV encephalitis is also associated with blindness. Congenital parvovirus is associated with fetal myocarditis and heart failure.[7] Complications of congenital HBV include progression to fulminant hepatitis or, rarely, chronic HBV infection. Congenital HCV carries a high risk of progression to chronic HCV leading to cirrhosis and an increased risk of hepatocellular carcinoma.[7]
HEV infection is associated with a significant risk of fulminant hepatic failure and death in pregnant women compared to the general population.[7] Untreated bacterial vaginosis is associated with an increased risk of preterm birth, chorioamnionitis, and endometritis.[46] Chorioamnionitis is associated with an increased risk of preterm birth, neonatal sepsis, interventricular hemorrhage, periventricular leukomalacia, and cerebral palsy.[10]
Data regarding the relationship between chorioamnionitis and bronchopulmonary dysplasia or respiratory distress syndrome is mixed.[10] Maternal complications from chorioamnionitis include a 2 to 3-fold increase in the risk of cesarean section and its associated complications as well as increased risk of endometritis, wound infection, postpartum hemorrhage, bacteremia and sepsis, and postpartum hemorrhage.[58][59]
Deterrence and Patient Education
There is a growing anti-vaccination movement that has resulted in the resurgence of diseases that were previously near-eradication. This movement is based on a paper published by Andrew Wakefield and his colleagues in 1998, which correlated MMR vaccination with the development of autism.[60] While this paper has been retracted and its evidence repeatedly debunked, public opinion of vaccination and skepticism of the medical establishment is still pervasive.[4][61]
Consequently, many women of child-bearing age are currently unvaccinated and at increased risk for antepartum infections. Screening for vaccination concerns at appointments can help identify at-risk individuals.[62] While this is best done before the patient becoming pregnant, discussing and addressing vaccination concerns in pregnant patients can not only protect subsequent pregnancies through vaccination of the patient but also encourage the patient to vaccinate her children, providing additional protection for the general population and future pregnant patients.[62][63][64]
HIV, HSV, and CMV can be vertically transmitted through breast milk.[7] HIV-positive mothers should not breastfeed to prevent vertical transmission of the virus.[65][66][65] As this is often difficult for these mothers, they should also be offered counseling and support. Mothers with known HSV but no active breast lesions may breastfeed but should be counseled to discontinue breastfeeding if any lesions develop on the breast to prevent spread to the infant.[66] There are no recommendations against breastfeeding for mothers that are CMV-seropositive. This is because otherwise, healthy infants who become infected after birth generally show minimal to no symptoms. However, mothers should be counseled that infants born at less than 30 weeks gestation and less than 1500 g birth weight are at increased risk of developing a sepsis-like syndrome if infected with CMV.[67]
Medical providers should help mothers weigh the risk of CMV transmission versus the benefits of breastfeeding in these cases. Freezing and pasteurization of breast milk can decrease the risk of transmission in these cases.
Pregnant women should also be instructed to avoid travel to locations where HEV and Zika virus are endemic and to avoid high-risk behaviors that increase the risk of contracting syphilis, HIV, HSV, HBV, and HCV.
Enhancing Healthcare Team Outcomes
Improved outcomes in antepartum infections may be achieved by targeting surmountable barriers to care. These include increased community education in prevention, provider education in risk minimization, and increased vaccination. Providers should seize all opportunities to provide patients with anticipatory care, including recommendations on safe sex practices and vaccination, which can help prevent antepartum infections. Congenital rubella, varicella, and hepatitis B can be prevented through maternal immunization.
Barriers to this include vaccine availability, provider failure to offer the vaccine, and vaccine skepticism. The use of standing orders for indicated vaccinations is associated with increased coverage and efficiency in vaccine administration in obstetrics and gynecology.[68][69] Additionally, ACOG recommends the institution of a standardized system for assessing a patient's immunization status at each visit and the use of electronic or paper prompts to remind providers which patients need vaccinations.[69]
Unfortunately, data to support an effective communication strategy for changing patient skepticism towards vaccination and increase vaccination uptake is lacking.[63] Education in risk minimization for providers is likewise paramount. For example, internal monitoring during labor should be avoided unless necessary, and vaginal examinations should be limited to decrease the risk of chorioamnionitis.