Issues of Concern
Transport Regulations
The packaging and shipping of infectious substances are under strict regulation as the potential consequences of exposure during transport can be devastating for both the environment and those exposed. The Committee of Experts created the UN Model Regulations on the Transport of Dangerous Goods (UNCETDG) to provide recommendations for the safe transport of dangerous goods.[2] The goal of the UN Model Regulations is to provide a foundation for transportation recommendations to create uniformity on the national and international levels.
The UN Model Regulations may require further modifications or additions by local governments and international organizations, depending on the full requirements for transport. Companies involved in transporting dangerous goods, such as airlines or couriers, may also enforce additional requirements. Also, international modal agreements created by international law provide guidelines for all acceptable modes of transportation, including air, rail, road, sea, and post. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022] Lab personnel should refer to the guidelines specific to the necessary form of transportation when preparing an infectious substance for shipment.
Transportation Stakeholders and Training
Federal hazardous materials transportation law states that all persons involved in hazardous materials' packaging and shipping process qualify as transportation stakeholders and must receive the necessary training. Those who receive training are referred to as hazmat employees/workers. A hazmat employer is any person or organization involved in transporting hazardous materials, including but not limited to shipping, transportation, manufacturing, certifying, and repairing. Hazmat training must include developing competencies in general awareness/familiarization, function-specific, safety, security awareness, security training, and driving training for those driving vehicles.[5] [See U.S. Department of Transportation. (2016, October 1). Hazmat Transportation Training Requirements. Pipeline and Hazardous Materials Safety Administration. Retrieved February 10, 2022] Training is crucial as lab workers are always at risk of self-exposure, and improper biocontainment increases the risk of environmental exposure.[6]
Employees can receive training under the Occupational Safety and Health Administration (OSHA), Environmental Protection Agency (EPA), or any other federal or international agency to satisfy the required competencies. Training should be completed within 90 days of employment and must be renewed every 3 years. Hazmat employees must keep training records that include the employee's name, training completion date, training details, hazmat trainer information, and training certification. Records should be kept for 3 years after the most recent training and up to 90 days after an employee leaves. Employees who have not fully completed training can only work under the direct supervision of a fully trained hazmat employee. [See U.S. Department of Transportation. (2016, October 1). Hazmat Transportation Training Requirements. Pipeline and Hazardous Materials Safety Administration. Retrieved February 10, 2022]
Classification System
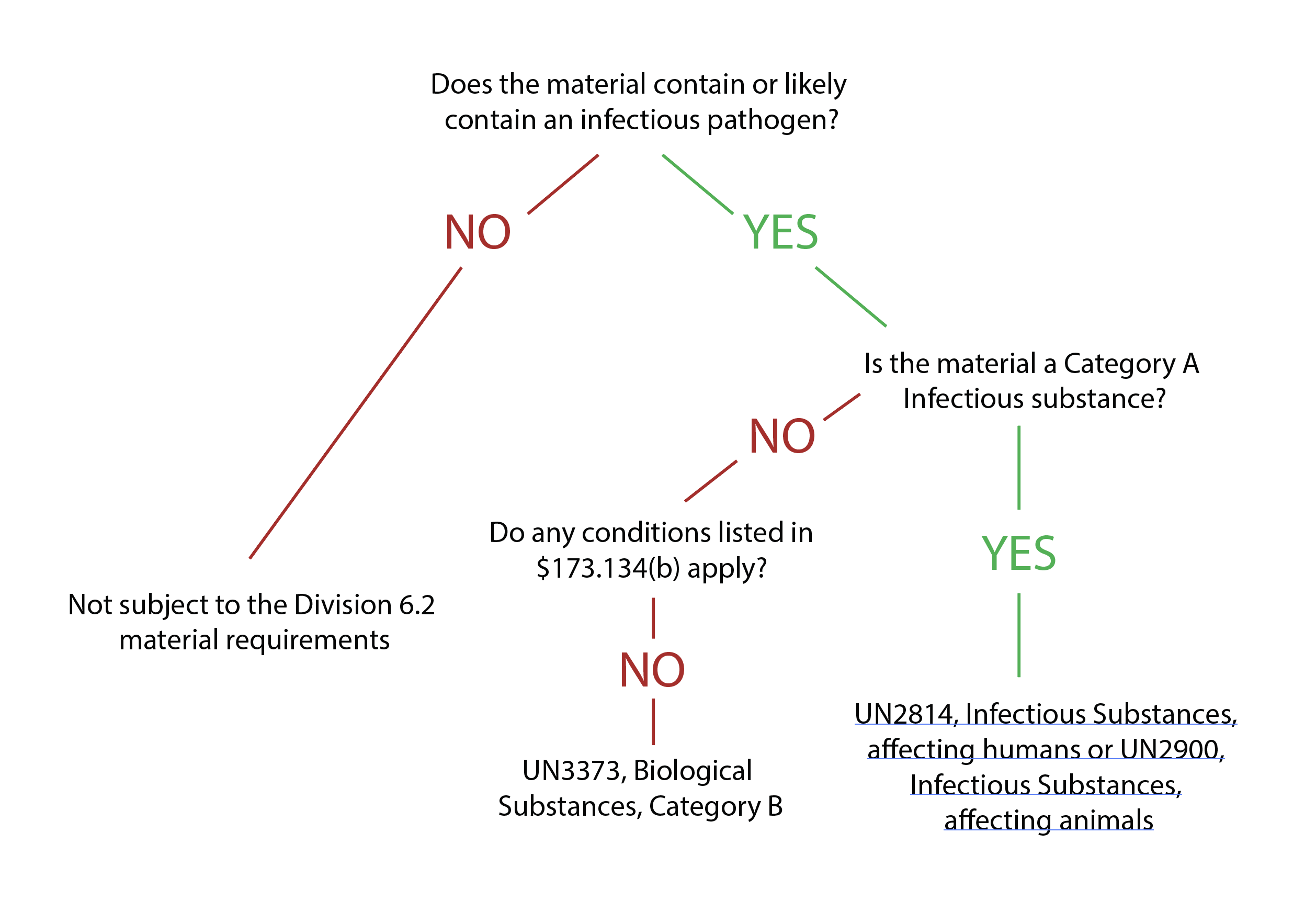
Infectious substances must be correctly defined for transport before the packaging process can begin. Materials that qualify as infectious substances include cultures, patient specimens, biological products, medical or clinical wastes, and medical devices or equipment. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022] These materials are classified under Category A or B to determine the proper packaging and shipping methods.
Category A classifies an infectious substance as one that can result in permanent disability or life-threatening disease in healthy humans or animals upon exposure.[2][3] Classification requires knowledge of the medical history of the source of the material or the use of professional judgment. Some examples of Category A substances include cultures of Mycobacterium tuberculosis, Human immunodeficiency virus (HIV), Coccidioides immitis, and the Ebola virus. A more complete list can be found towards the end of this paper. [See U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022]
The proper shipping names and identification numbers for Category A substances include:
- UN2814, Infectious substances affecting humans
- UN2900, Infectious substances affecting animals
- UN3549 regulated medical waste not otherwise specified (Category A substances produced from the medical treatment of humans or animals)[See U.S. Department of Transportation. (2020, April 28) Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022] [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022].[7]
Category B classifies an infectious substance as incapable of causing permanent disability or life-threatening disease in healthy humans or animals. Category B substances do not meet the Category A criteria. Category B substances include Mycobacterium tuberculosis and HIV clinical specimens, Arbovirus, and hepatitis A, B, and C.[5] Category B includes materials transported for diagnostic purposes.[2]
The proper shipping names and identification numbers for Category B substances include:
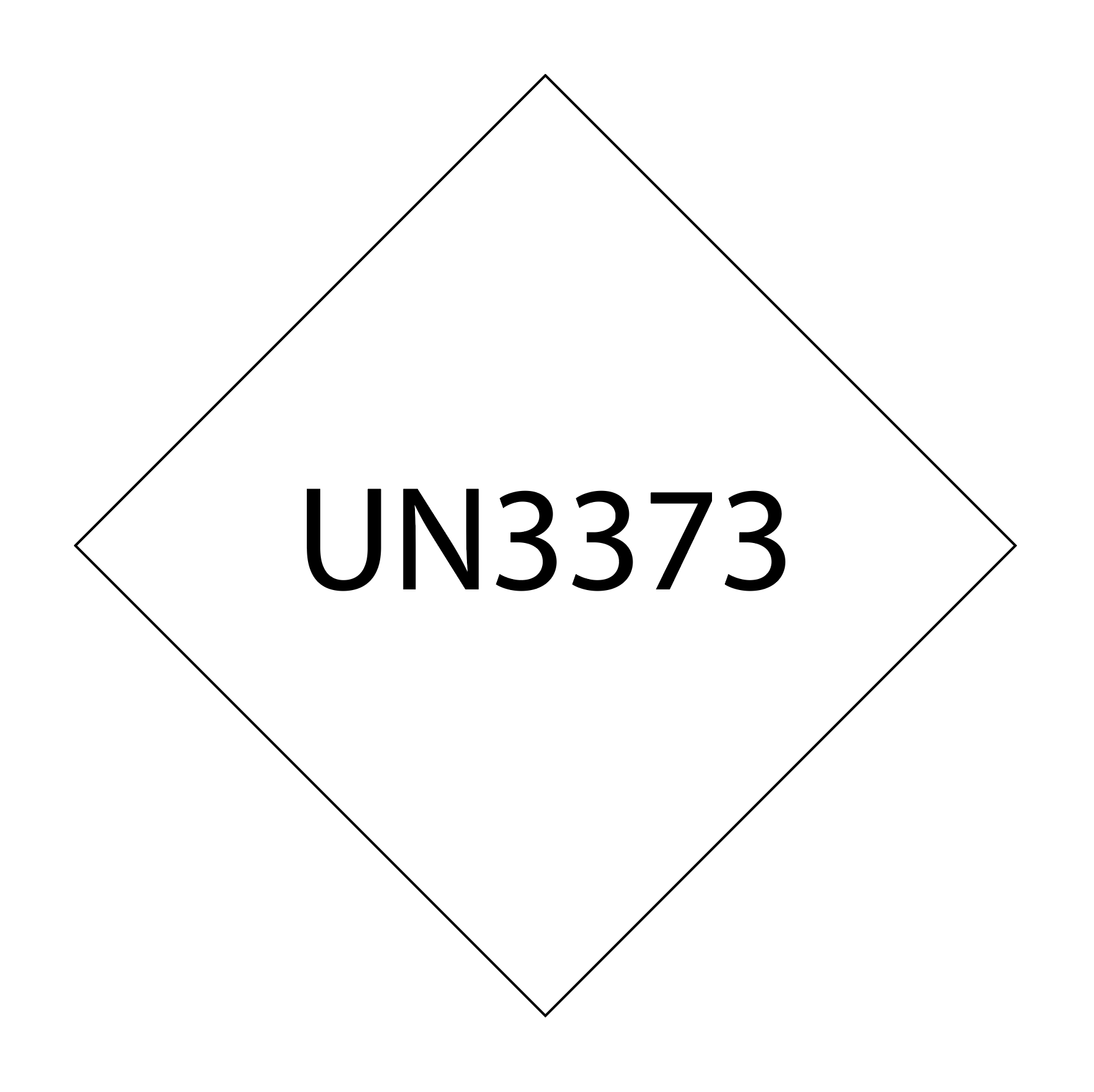
- UN3733, biological substances, Category B
- UN3291, regulated medical waste, n.o.s. (Category B substances produced from the medical treatment of humans or animals) [See U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022] [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
Exceptions
Despite establishing a classification system, it may not always be clear whether a substance is infectious and requires strict regulations for packaging and shipping. Furthermore, exceptions exist for transporting medical or laboratory waste that does not meet the criteria of category A or B infectious substances. Section 173.134(b) of the HMR provides exceptions for materials not bound by the requirements of infectious substances. [See U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022]
Substances exempt from strict regulations do not contain biological agents, or any biological agents present are incapable of causing disease. Therefore, these materials do not have to follow transport regulations if specific protocols are followed.
Examples of exempt materials include:
- Cultures that are non-pathogenic to humans or animals.
- Patient specimens from blood screening or tests that use a dried blood spot.
- Biological products include blood products for transfusion or organ transplants.
- Medical/clinical waste that has been decontaminated with autoclaving or incineration.
- Medical equipment free of contaminated liquid
- Environmental samples, such as food, soil, or water, are shipped for research and cannot infect humans or animals.
If transported by air, these exemptions are subject to modal regulations requiring a triple packaging system. Liquids also require the use of absorbent material. Besides the triple packaging system, these substances are exempt from all other infectious substances regulations. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
The IATA and United States Postal Service (USPS) define human or animal samples transported for routine testing unrelated to diagnosing an infectious disease as exempt specimens. This includes specimens for drug, alcohol, cholesterol, blood glucose level, prostate-specific antibody (PSA), kidney function, liver function, pregnancy, and noninfectious disease testing.
IATA requirements for exempt specimens include:
- Triple packaging
- Outer package with one surface with a minimum dimension of 100 mm x 100 mm
- Outer package able to survive a 4-foot drop test
USPS requirements for exempt specimens include:
- Triple packaging
- Outer package with one surface with a minimum dimension of 100 mm x 100 mm
- Outer package able to survive a 4-foot drop test
- Quantity limit of 500 mL for the primary receptacle
- Quantity limit of 500 mL secondary container [See Laboratory Continuing Education. (2013). IATA and US Postal Service Exempt Specimens. Lab CE. Retrieved February 10, 2022]
Shipment Requirements
Infectious substances must meet the packaging, marking, and labeling requirements for transport (see Image. Packaging Flow Chart). Packaging infectious substances involves a triple packaging system consisting of a primary receptacle, secondary container, and rigid outer packaging. [See Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP). (2016, December 1); Packaging and transporting infectious substances. Centers for Disease Control and Prevention. Retrieved January 2, 2022]
The primary receptacle contains infectious material and must be leakproof. Liquids require packaging with an absorbent material capable of absorbing the entirety of the specimen if a leak occurs.
The secondary container must also be leakproof and enclose the primary receptacle. Cushioning may also be necessary to secure a primary receptacle within the secondary container firmly. Multiple primary receptacles can be placed in the secondary container if the transported substances are of the same class. If the receptacles are fragile, each must be individually wrapped or supplemented with cushioning to prevent contact. Documentation should be placed between the secondary container and rigid outer packaging.
The third layer, the rigid outer packaging, must protect the secondary container from damage. It must have the appropriate dimensions and strength to contain the primary and secondary containers. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
In addition to the triple packaging system, infectious substances may require a preservative to maintain integrity throughout transport. Some examples of packaging preservatives include coolants and stabilizers. A coolant/refrigerant is used to maintain a cool temperature during transportation. Coolants often also qualify as dangerous goods and are subject to packaging requirements.
The requirements of packaging an infectious substance with a coolant include:
- Packaging that can maintain integrity at the temperature of the coolant
- Coolant should be placed between the secondary and outer package
- Hazmat employers should be trained in handling coolants
- Adequate ventilation for the cargo transport unit
- Necessary marking and documentation for coolants
Coolants frequently used include wet or dry ice and liquid nitrogen. Dry ice and liquid nitrogen are considered dangerous goods. The proper shipping name for dry ice is "dry ice" or "carbon dioxide, solid." The UN number is UN 1845. The proper shipping name for liquid nitrogen is "nitrogen refrigerated liquid." The UN number is UN 1977.
A stabilizer is a chemical used to prevent the degradation or neutralize the hazards of an infectious substance. Stabilizers frequently used include sorbitol, fetal bovine serum, alcohol, and formaldehyde. Stabilizers can also qualify as dangerous goods and must be added appropriately to the infectious substance's primary receptacle. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
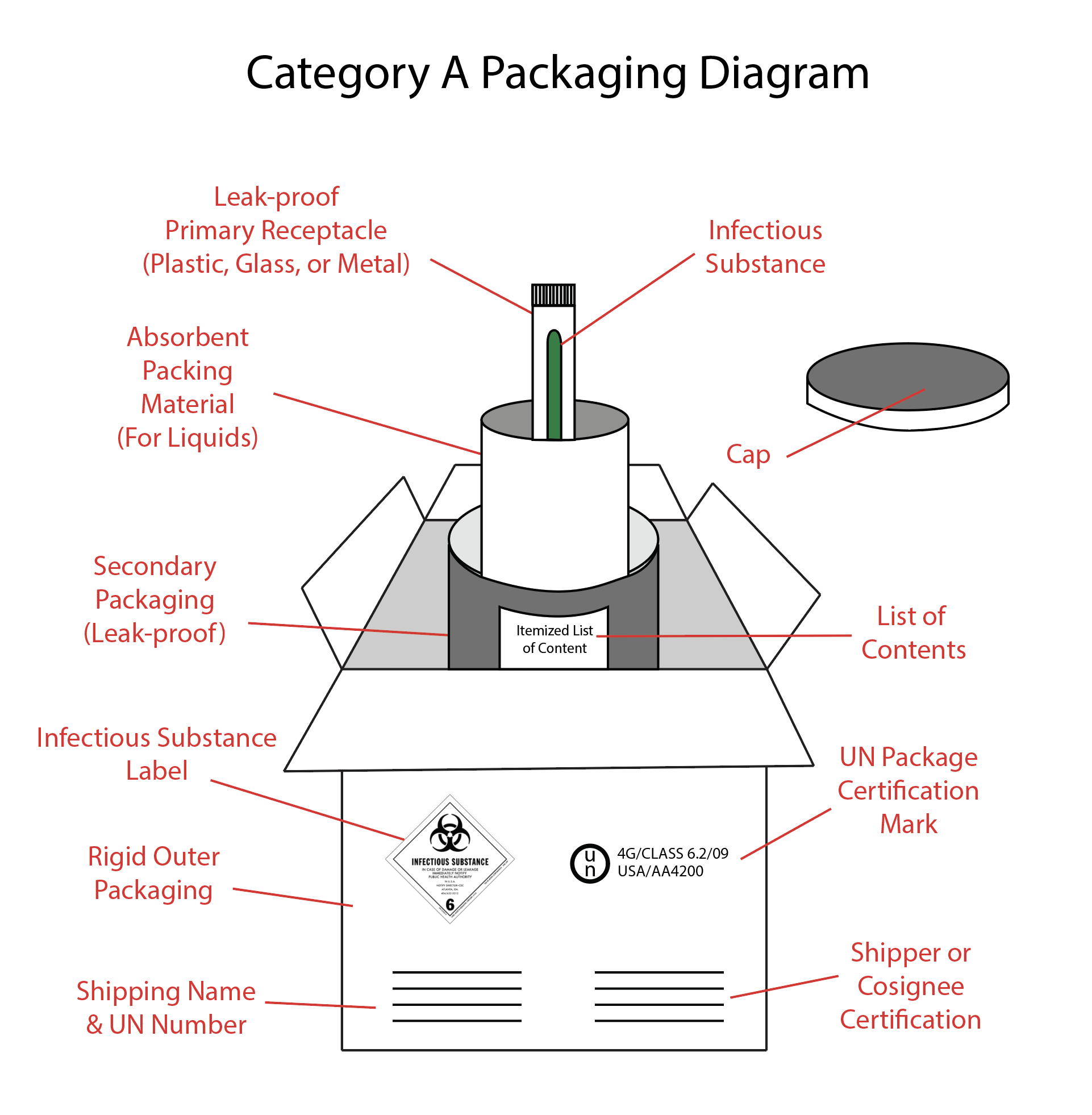
Category A Packaging Requirements
Category A infectious substance packaging requirements (see Image. Category A Packaging) can be found in Section 173.196 of HMR and must:
- Meet the standards of CFR §173.609
- Consist of triple packaging, including leakproof primary containers, leakproof secondary containers, and rigid outer packaging.
- Have UN certification markers on the outer packaging
Primary or secondary packaging is indicated for temperatures ranging from -40° C to 55° C. Shipments transported at these temperatures must be glass, metal, or plastic. A heat seal, skirted stopper, or metal crimp seals should be used when necessary to form a leakproof seal. Screw caps must be secured with paraffin sealing tape or a manufactured locking closer. The outer packaging must be rigid and have at least one surface with a minimum dimension of 100 mm x 100 mm (3.9 in x 3.9 in). ) [See U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022]
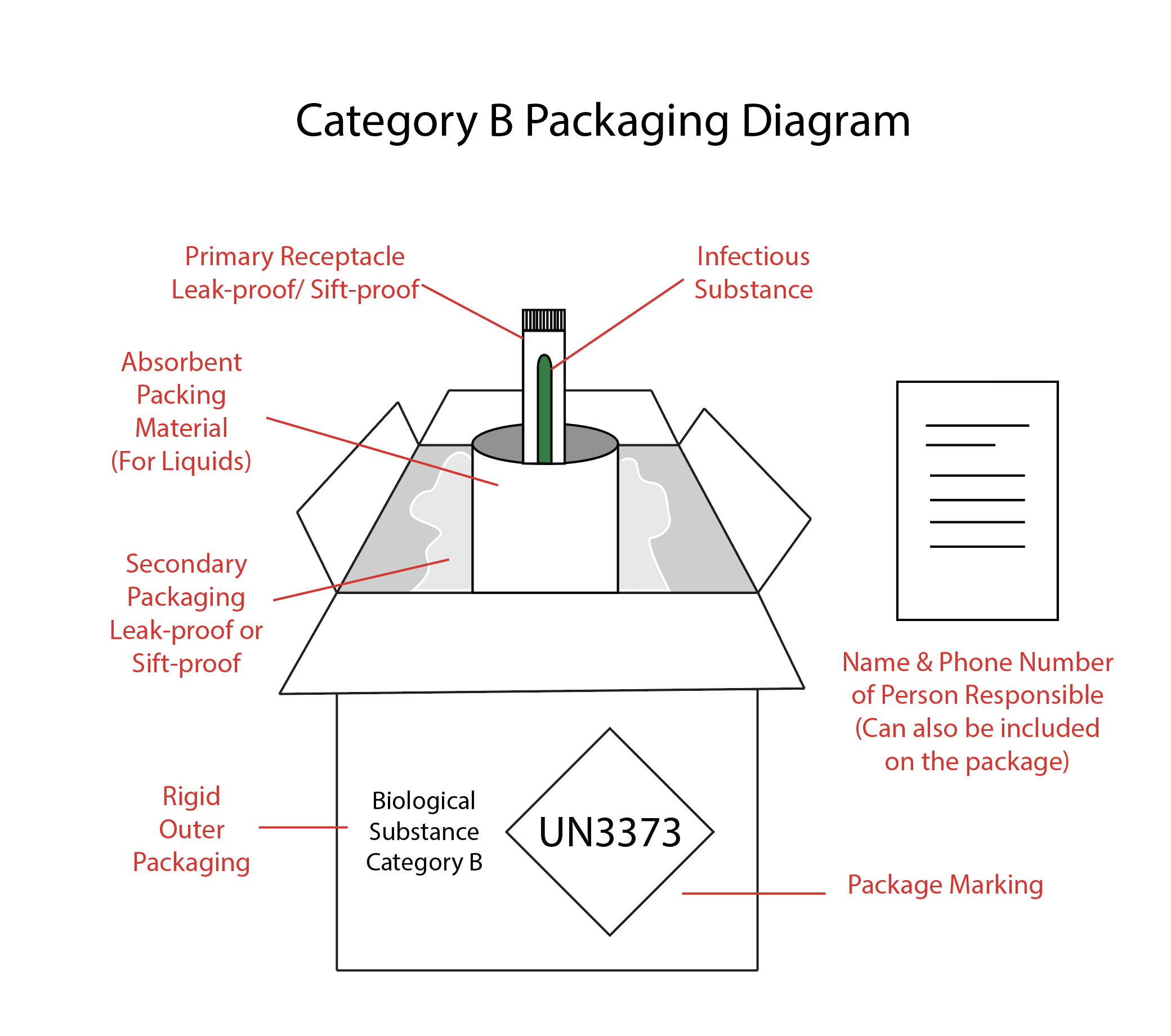
Category B Packaging Requirements
Category B infectious substances do not have to follow the requirements of HMR (see Image. Category B Packaging) but must:
- Meet the standards of CFR §173.199
- Consist of the outer package marked with "Biological substances Category B" adjacent to the proper shipping name.
- Have the UN3373 package marking
The primary or secondary package must withstand an internal pressure that produces a pressure difference of 95 kPa or higher. The secondary or outer packaging should be rigid for surface transport (road, train, or ship). If the secondary packaging is soft, the outer packaging must be rigid, and vice versa. For air transport, the outer packaging must always be rigid. The packaging must also pass a 1.2-meter drop test to ensure it is strong enough to withstand transport. Medical or clinical waste categorized as Category B substances (UN3921) does not have to follow the triple packaging system. [See U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022]
Marking and Labeling
After an infectious substance has been packaged, lab personnel must place the correct marks and labels that provide information about the package, the infectious substance, and packaging standards (see Images. Packaging Label Example and Package Marking Example).
Packages containing infectious substances should be marked with the following:
- The sender's name and address
- The consignor's (shipper's) name and address
- The consignee's (receiver's) name and address
- UN number and proper shipping name of the infectious substance
- If a coolant is used, its UN number and proper shipping name must be included, followed by 'AS COOLANT.' The net quantity of the coolant should also be specified.
Category A marking should include the UN packaging symbol and certification and the name and contact information of the person responsible for the shipment. Category B marking should include the UN3373 mark with the correct dimensions, a visible mark on the outer packaging, the proper shipping name, and biological substance category B.
In addition to correct markers, packages may require hazard labels, handling labels, or both. (see Image. Hazard Communication Standard Pictogram) Hazard labels consist of a diamond shape with minimum dimensions of 100 mm x 100 mm. Each dangerous substance should have one hazard label; a package may have multiple labels. Handling labels describe how the package should be handled during transport. Examples include orientation arrows, Cargo Aircraft Only (CAO) labels, and cryogenic liquid warning labels. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
Documenting Shipments
Packages carrying infectious substances require documentation regarding the contents and package preparation. The UN Model Regulations require a Dangerous Goods Transport Document (DGTD) for all Category A substances. The DGTD must contain the sender and receiver information, the date the document was signed, a description of the contents, the NET quantity of dangerous goods, handling requirements, emergency response information, and certification/shipper's declaration. Category B substances do not require a DGTD. Hazardous goods transported internationally by air require an air waybill in addition to the DGTD. An air waybill is a requirement for transporting all goods by air. The "Handling Information" box and the "Nature and Quantity of Goods" box must be filled on the airway bill to ship dangerous goods. [See World Health Organization. (2021, February 25). Guidance on regulations for the transport of infectious substances 2021-2022. World Health Organization. Retrieved January 2, 2022]
Emergency Response Information
All employees involved in the hazardous materials transport chain must know the initial emergency response procedures and whom to notify in the event of an incident.[8] The shipper and consignee (the recipient) must be capable of providing specific information regarding a shipment.
Shipments of Category A infectious substances must also include contact information for the responsible person and a 24-hour emergency contact. The first requirement is the name and telephone number of the person responsible for the shipment. This information should be marked on the outside of the package. The responsible person could be the shipper, receiver, or a third party. The package will also often bear the shipper's name and number and the recipient's name and number.
The second requirement is a 24-hour emergency response telephone number occupied by someone knowledgeable about the emergency response requirements and incident mitigation information for the shipped material. This number is not marked on the package but must be included in the additional handling information box on the Shipper's Declaration.[9]
Individuals should use the 24-hour emergency contact number listed on the Shipper's Declaration for emergency response inquiries about an incident involving a Category A infectious substance shipment. However, the 24-hour emergency contact and the responsible person should know about the package's contents. They should be able to provide the inquiring person with detailed risk assessment information on the material, such as the routes of exposure and basic exposure response procedures.[8] Examples of basic response procedures include washing an affected area of the body for 15 minutes with soap and water or an eyewash, notifying supervisors, seeking immediate medical assistance, isolating a spill with caution tape (or other means), and keeping others informed about the spill or out of the spill area until qualified emergency responders arrive for mitigation.[10]
Infectious Substance Spill Management
In the event of an infectious substance spill, prompt and effective response is crucial to minimize risks and ensure the safety of all individuals involved.[11] The initial response guidelines dictate that individuals should avoid inhaling airborne material by swiftly leaving the room, removing gloves, and notifying others to evacuate. It is essential to close the door behind to contain the spill and prominently display a warning sign to prevent accidental entry. Subsequently, any contaminated clothing must be carefully removed, exposed areas turned inward and disposed of in a designated biohazard bag. Following this, thoroughly washing all exposed skin with soap and water is recommended to eliminate any potential contamination. Additionally, it is imperative to inform the supervisor of the incident and seek assistance from the safety office to ensure a comprehensive and coordinated response. These guidelines are designed to be swift, systematic, and aimed at safeguarding the well-being of individuals and maintaining a secure environment.[12]
The procedure for cleaning up an infectious substance spill is a critical aspect of laboratory safety protocols.[13] Immediately after a spill, it is imperative to allow aerosols to disperse for a minimum of 30 minutes before reentering the laboratory to ensure a safe environment. The next step involves assembling the necessary clean-up materials, including disinfectant, paper towels, biohazard bags, and forceps. Before initiating the clean-up, it is essential to don protective clothing, such as a lab coat, face protection, utility gloves, and booties. Depending on the spill's nature, a HEPA-filtered respirator may be preferable over a surgical mask.[14]
To contain and neutralize the spill, the affected area should be covered with disinfectant-soaked towels and disinfectant poured carefully around the spill to avoid enlarging the contaminated zone. A contact time of at least 20 minutes is recommended. Sharp objects should be handled with forceps and disposed of in a sharps container. Surrounding areas should be wiped down with disinfectant to address potential splashes. The following steps involve soaking up the disinfectant and spill, placing the materials into a biohazard bag, and spraying the area with a 10% household bleach solution. After a 15-minute contact, the area should be allowed to air-dry or wiped down with disinfectant-soaked towels. Contaminated paper towels and protective clothing are placed into a biohazard bag for autoclaving. Finally, hands and exposed skin should be thoroughly washed with soap and water to ensure personal hygiene.[10] This comprehensive procedure is designed to systematically and safely address infectious substance spills, minimizing risks to laboratory personnel and maintaining a secure research environment.
List of Category A Infectious Substances
Category A substances affecting humans (UN 3814). This list is adapted from [U.S. Department of Transportation. (2020, April 28). Transporting infectious substances safely. Pipeline and Hazardous Materials Safety Administration. Retrieved January 2, 2022]
- Bacillus anthracis cultures
- Brucella abortus cultures
- Brucella melitensis cultures
- Brucella suis cultures
- Burkholderia mallei—Pseudomonas mallei—Glanders cultures only
- Burkholderia pseudomallei—Pseudomonas pseudomallei cultures
- Chlamydia psittaci—avian strains cultures
- Clostridium botulinum cultures
- Coccidioides immitis cultures
- Coxiella burnetii cultures
- Crimean-Congo hemorrhagic fever virus
- Dengue virus cultures
- Eastern equine encephalitis virus cultures
- Escherichia coli, verotoxigenic cultures
- Ebola virus
- Flexal virus
- Francisella tularensis cultures
- Guanarito virus
- Hantaan virus
- Hantaviruses causing hemorrhagic fever with renal syndrome
- Hendra virus
- Herpes B virus cultures
- Human immunodeficiency virus cultures
- Highly pathogenic avian influenza virus cultures
- Japanese Encephalitis virus cultures
- Junin virus
- Kyasanur forest disease virus
- Lassa virus
- Machupo virus
- Marburg virus
- Monkeypox virus
- Mycobacterium tuberculosis cultures only
- Nipah virus
- Omsk hemorrhagic fever virus
- Poliovirus cultures
- Rabies and other lyssa viruses cultures
- Rickettsia prowazekii cultures
- Rickettsia rickettsia cultures
- Rift Valley fever virus cultures
- Russian spring-summer encephalitis virus cultures
- Sabia virus
- Shigella dysenteriae type I cultures
- Tick-borne encephalitis virus cultures
- Variola virus
- Venezuelan equine encephalitis virus (cultures
- Vesicular stomatitis virus cultures
- West Nile virus cultures
- Yellow fever virus cultures
- Yersinia pestis cultures
Category A Substances Affecting Animals (UN2900)
- African swine fever virus cultures
- Avian paramyxovirus type 1—Velogenic Newcastle disease virus cultures
- Classical swine fever virus cultures
- Foot and mouth disease virus cultures
- Lumpy skin disease virus cultures
- Mycoplasma mycoides—Contagious bovine pleuropneumonia cultures
- Peste des petits ruminants virus cultures
- Rinderpest virus cultures
- Sheep-pox virus cultures
- Goatpox virus cultures
- Swine vesicular disease virus cultures