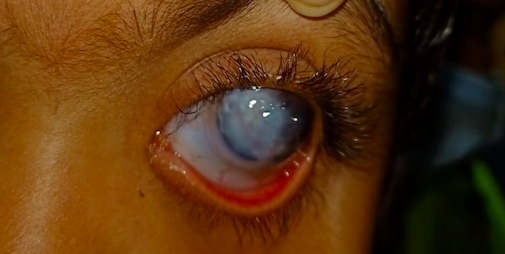
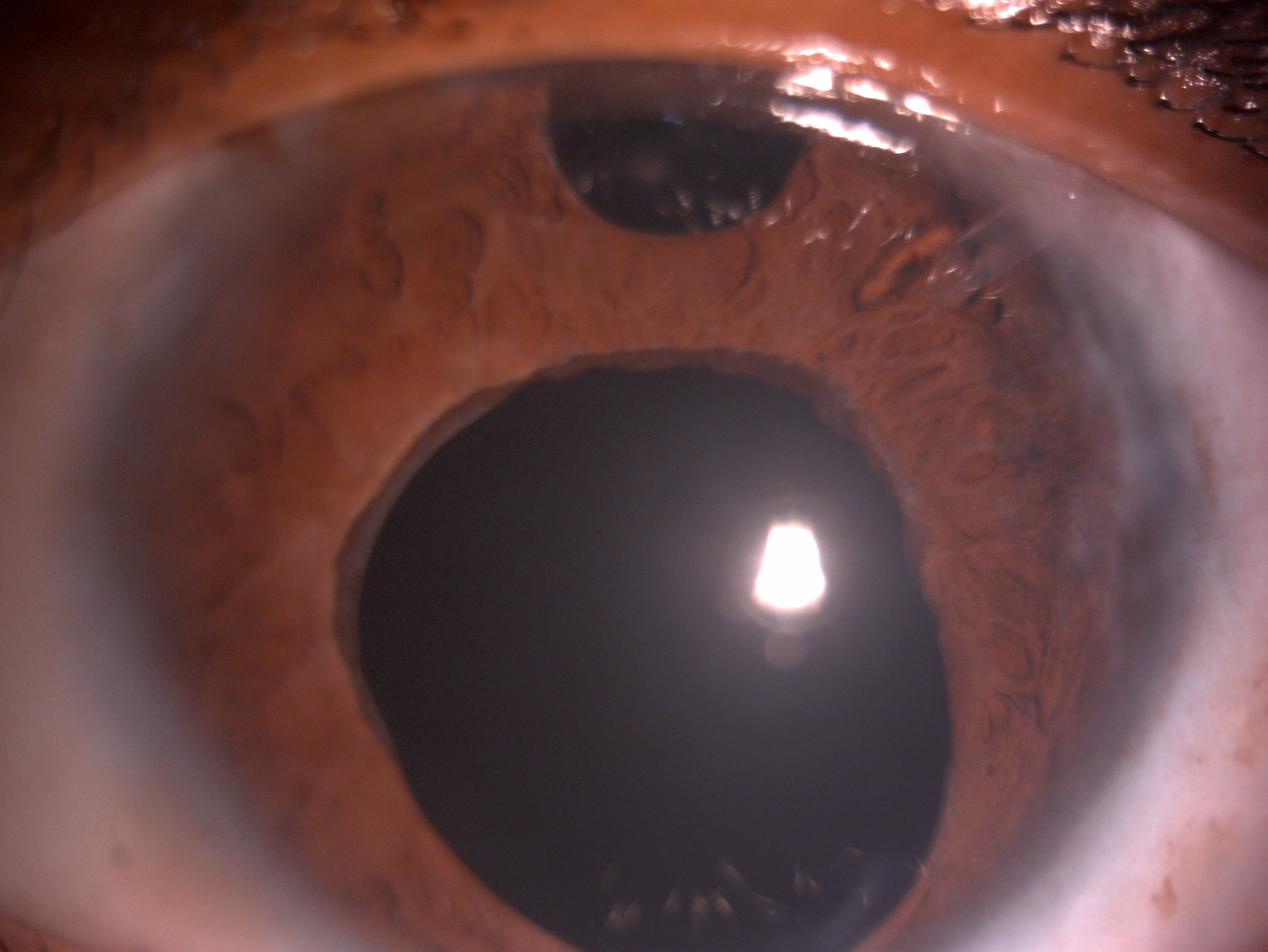
[1]
Reis LM, Seese SE, Costakos D, Semina EV. Congenital anterior segment ocular disorders: Genotype-phenotype correlations and emerging novel mechanisms. Progress in retinal and eye research. 2024 Sep:102():101288. doi: 10.1016/j.preteyeres.2024.101288. Epub 2024 Aug 2
[PubMed PMID: 39097141]
[2]
Torné O, Oikawa K, Teixeira LBC, Kiland JA, McLellan GJ. Trabecular Meshwork Abnormalities in a Model of Congenital Glaucoma Due to LTBP2 Mutation. Investigative ophthalmology & visual science. 2024 Oct 1:65(12):28. doi: 10.1167/iovs.65.12.28. Epub
[PubMed PMID: 39432401]
[3]
Gurpinar A, Niyaz L, Ariturk N. Long-term follow-up results and visual outcomes of childhood glaucoma in the black sea region of turkey. International ophthalmology. 2024 Aug 29:44(1):360. doi: 10.1007/s10792-024-03275-7. Epub 2024 Aug 29
[PubMed PMID: 39207647]
[4]
Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA. Primary congenital glaucoma: An updated review. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2019 Oct-Dec:33(4):382-388. doi: 10.1016/j.sjopt.2019.10.002. Epub 2019 Nov 7
[PubMed PMID: 31920449]
[5]
Kaushik S, Senthil S, Gupta V, Balekudaru S, Dubey S, Ali H, Mandal AK, Indian Childhood Glaucoma Study (ICGS) Group. Profile of Newly Diagnosed Childhood Glaucoma in India: Indian Childhood Glaucoma Study (ICGS) Group 1. Ophthalmology. Glaucoma. 2024 Jan-Feb:7(1):54-65. doi: 10.1016/j.ogla.2023.07.004. Epub 2023 Jul 15
[PubMed PMID: 37454975]
[6]
Choe S, Kim YK, Ha A. Nationwide incidence of and risk factors for undergoing incisional glaucoma surgery following infantile cataract surgery. Scientific reports. 2024 Jul 15:14(1):16286. doi: 10.1038/s41598-024-66559-z. Epub 2024 Jul 15
[PubMed PMID: 39009616]
[7]
Selvan H, Gupta S, Wiggs JL, Gupta V. Juvenile-onset open-angle glaucoma - A clinical and genetic update. Survey of ophthalmology. 2022 Jul-Aug:67(4):1099-1117. doi: 10.1016/j.survophthal.2021.09.001. Epub 2021 Sep 16
[PubMed PMID: 34536459]
Level 3 (low-level) evidence
[8]
Hoskins HD Jr, Shaffer RN, Hetherington J. Anatomical classification of the developmental glaucomas. Archives of ophthalmology (Chicago, Ill. : 1960). 1984 Sep:102(9):1331-6
[PubMed PMID: 6477252]
[9]
Pan Y, Iwata T. Exploring the Genetic Landscape of Childhood Glaucoma. Children (Basel, Switzerland). 2024 Apr 9:11(4):. doi: 10.3390/children11040454. Epub 2024 Apr 9
[PubMed PMID: 38671671]
[10]
Mandal AK, Raghavachary C, Peguda HK. Haab's Striae. Ophthalmology. 2017 Jan:124(1):11. doi: 10.1016/j.ophtha.2016.07.002. Epub
[PubMed PMID: 27993263]
[11]
Knight LSW, Ruddle JB, Taranath DA, Goldberg I, Smith JEH, Gole G, Chiang MY, Willett F, D'Mellow G, Breen J, Qassim A, Mullany S, Elder JE, Vincent AL, Staffieri SE, Kearns LS, Mackey DA, Luu S, Siggs OM, Souzeau E, Craig JE. Childhood and Early Onset Glaucoma Classification and Genetic Profile in a Large Australasian Disease Registry. Ophthalmology. 2021 Nov:128(11):1549-1560. doi: 10.1016/j.ophtha.2021.04.016. Epub 2021 Apr 20
[PubMed PMID: 33892047]
[12]
Haddad A, Ait Boujmia OK, El Maaloum L, Dehbi H. Meta-analysis of CYP1B1 gene mutations in primary congenital glaucoma patients. European journal of ophthalmology. 2021 Nov:31(6):2796-2807. doi: 10.1177/11206721211016308. Epub 2021 May 21
[PubMed PMID: 34020567]
Level 1 (high-level) evidence
[13]
Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. American journal of human genetics. 2009 May:84(5):664-71. doi: 10.1016/j.ajhg.2009.03.017. Epub 2009 Apr 9
[PubMed PMID: 19361779]
[14]
Suri F, Yazdani S, Elahi E. LTBP2 knockdown and oxidative stress affect glaucoma features including TGFβ pathways, ECM genes expression and apoptosis in trabecular meshwork cells. Gene. 2018 Oct 5:673():70-81. doi: 10.1016/j.gene.2018.06.038. Epub 2018 Jun 14
[PubMed PMID: 29908281]
[15]
Sarfarazi M, Akarsu AN, Hossain A, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS. Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995 Nov 20:30(2):171-7
[PubMed PMID: 8586416]
[16]
Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. The Journal of clinical investigation. 2010 Sep:120(9):3064-72. doi: 10.1172/JCI43085. Epub 2010 Sep 1
[PubMed PMID: 20811162]
[17]
Kumar A, Han Y, Oatts JT. Genetic changes and testing associated with childhood glaucoma: A systematic review. PloS one. 2024:19(2):e0298883. doi: 10.1371/journal.pone.0298883. Epub 2024 Feb 22
[PubMed PMID: 38386645]
Level 1 (high-level) evidence
[18]
Lim SH, Tran-Viet KN, Yanovitch TL, Freedman SF, Klemm T, Call W, Powell C, Ravichandran A, Metlapally R, Nading EB, Rozen S, Young TL. CYP1B1, MYOC, and LTBP2 mutations in primary congenital glaucoma patients in the United States. American journal of ophthalmology. 2013 Mar:155(3):508-517.e5. doi: 10.1016/j.ajo.2012.09.012. Epub 2012 Dec 4
[PubMed PMID: 23218701]
[19]
Tamçelik N, Atalay E, Bolukbasi S, Çapar O, Ozkok A. Demographic features of subjects with congenital glaucoma. Indian journal of ophthalmology. 2014 May:62(5):565-9. doi: 10.4103/0301-4738.126988. Epub
[PubMed PMID: 24881602]
[20]
Genĉík A. Epidemiology and genetics of primary congenital glaucoma in Slovakia. Description of a form of primary congenital glaucoma in gypsies with autosomal-recessive inheritance and complete penetrance. Developments in ophthalmology. 1989:16():76-115
[PubMed PMID: 2676634]
[21]
Yu-Wai-Man C, Arno G, Brookes J, Garcia-Feijoo J, Khaw PT, Moosajee M. Primary congenital glaucoma including next-generation sequencing-based approaches: clinical utility gene card. European journal of human genetics : EJHG. 2018 Nov:26(11):1713-1718. doi: 10.1038/s41431-018-0227-y. Epub 2018 Aug 8
[PubMed PMID: 30089822]
[22]
deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma). Survey of ophthalmology. 1983 Jul-Aug:28(1):1-19
[PubMed PMID: 6353647]
Level 3 (low-level) evidence
[23]
Ohtake Y, Tanino T, Suzuki Y, Miyata H, Taomoto M, Azuma N, Tanihara H, Araie M, Mashima Y. Phenotype of cytochrome P4501B1 gene (CYP1B1) mutations in Japanese patients with primary congenital glaucoma. The British journal of ophthalmology. 2003 Mar:87(3):302-4
[PubMed PMID: 12598442]
[24]
Alabdulwahhab KM, Ahmad MS. Visual Impairment and Blindness in Saudi Arabia's School for the Blind: A Cross-Sectional Study. Clinical optometry. 2020:12():169-173. doi: 10.2147/OPTO.S265293. Epub 2020 Oct 7
[PubMed PMID: 33117027]
Level 2 (mid-level) evidence
[25]
Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, Feng L, Limviphuvadh V, Whisenhunt KN, Maurer-Stroh S, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Mauri L, Javadiyan S, Souzeau E, Zhou T, Hewitt AW, Kloss B, Burdon KP, Mackey DA, Allen KF, Ruddle JB, Lim SH, Rozen S, Tran-Viet KN, Liu X, John S, Wiggs JL, Pasutto F, Craig JE, Jin J, Quaggin SE, Young TL. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. The Journal of clinical investigation. 2016 Jul 1:126(7):2575-87. doi: 10.1172/JCI85830. Epub 2016 Jun 6
[PubMed PMID: 27270174]
[26]
BARKAN O. Pathogenesis of congenital glaucoma: gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. American journal of ophthalmology. 1955 Jul:40(1):1-11
[PubMed PMID: 14388087]
[27]
Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian journal of ophthalmology. 2011 Jan:59 Suppl(Suppl1):S148-57. doi: 10.4103/0301-4738.73683. Epub
[PubMed PMID: 21150027]
[28]
Mandal AK, Chakrabarti D, Gothwal VK. Approach to primary congenital glaucoma: A perspective. Taiwan journal of ophthalmology. 2023 Oct-Dec:13(4):451-460. doi: 10.4103/tjo.TJO-D-23-00104. Epub 2023 Oct 19
[PubMed PMID: 38249492]
Level 3 (low-level) evidence
[29]
Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in congenital glaucoma. American journal of ophthalmology. 1981 Oct:92(4):508-25
[PubMed PMID: 7294114]
[30]
García-Antón MT, Salazar JJ, de Hoz R, Rojas B, Ramírez AI, Triviño A, Aroca-Aguilar JD, García-Feijoo J, Escribano J, Ramírez JM. Goniodysgenesis variability and activity of CYP1B1 genotypes in primary congenital glaucoma. PloS one. 2017:12(4):e0176386. doi: 10.1371/journal.pone.0176386. Epub 2017 Apr 27
[PubMed PMID: 28448622]
[31]
Papadopoulos M, Cable N, Rahi J, Khaw PT, BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Investigative ophthalmology & visual science. 2007 Sep:48(9):4100-6
[PubMed PMID: 17724193]
[32]
Mandal AK. Acute Corneal Hydrops in Children with Primary Infantile Glaucoma: A Report of 31 Cases over 23 Years at the LVPEI. PloS one. 2016:11(6):e0156108. doi: 10.1371/journal.pone.0156108. Epub 2016 Jun 1
[PubMed PMID: 27249057]
Level 3 (low-level) evidence
[33]
Drechsler J, Lee A, Maripudi S, Kueny L, Levin MR, Saeedi OJ, Bazemore M, Karwoski B, Birdsong R, Martinez C, Jaafar MS, Yousaf S, Ahmed ZM, Madigan WP, Alexander JL. Corneal Structural Changes in Congenital Glaucoma. Eye & contact lens. 2022 Jan 1:48(1):27-32. doi: 10.1097/ICL.0000000000000844. Epub
[PubMed PMID: 34608027]
[34]
Gupta S, Mahalingam K, Singh A, Selvan H, Somarajan BI, Gupta V. Posterior corneal morphological changes in primary congenital glaucoma. Indian journal of ophthalmology. 2022 Jul:70(7):2571-2577. doi: 10.4103/ijo.IJO_317_22. Epub
[PubMed PMID: 35791159]
[35]
Sihota R, Mahalingam K, Maurya AK, Sharma A, Bukke AN, Dada T. Primary congenital glaucoma: An iridotrabeculodysgenesis? Indian journal of ophthalmology. 2024 Mar 1:72(3):328-334. doi: 10.4103/IJO.IJO_370_23. Epub 2023 Dec 15
[PubMed PMID: 38099353]
[36]
Walton DS. Chronic newborn primary congenital glaucoma with secondary lens subluxation. Journal of pediatric ophthalmology and strabismus. 2009 Jul-Aug:46(4):200, 231. doi: 10.3928/01913913-20090706-02. Epub
[PubMed PMID: 19645394]
[37]
Snehi S, Singh AK, Kaushik S. Acquired Lens Zonular Loss with Bean Pot Optic Disc Cupping in Congenital Glaucoma. Ophthalmology. Glaucoma. 2023 Mar-Apr:6(2):159. doi: 10.1016/j.ogla.2023.01.001. Epub 2023 Jan 27
[PubMed PMID: 36710134]
[38]
Gupta V, James MK, Singh A, Kumar S, Gupta S, Sharma A, Sihota R, Kennedy DJ. Differences in Optic Disc Characteristics of Primary Congenital Glaucoma, Juvenile, and Adult Onset Open Angle Glaucoma Patients. Journal of glaucoma. 2016 Mar:25(3):239-43. doi: 10.1097/IJG.0000000000000154. Epub
[PubMed PMID: 25265002]
[39]
Sihota R, Sidhu T, Agarwal R, Sharma A, Gupta A, Sethi A, Dada T, Pandey V. Evaluating target intraocular pressures in primary congenital glaucoma. Indian journal of ophthalmology. 2021 Aug:69(8):2082-2087. doi: 10.4103/ijo.IJO_3473_20. Epub
[PubMed PMID: 34304183]
[40]
Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmology clinics of North America. 2001 Sep:14(3):501-12
[PubMed PMID: 11705150]
[41]
Ramyashri S, Senthil S. Effect of Haab's striae on corneal parameters in primary congenital glaucoma. Indian journal of ophthalmology. 2024 Nov 1:72(11):1679-1680. doi: 10.4103/IJO.IJO_2958_23. Epub 2024 Oct 26
[PubMed PMID: 39462935]
[42]
Sihota R, Sidhu T, Dada T. The role of clinical examination of the optic nerve head in glaucoma today. Current opinion in ophthalmology. 2021 Mar 1:32(2):83-91. doi: 10.1097/ICU.0000000000000734. Epub
[PubMed PMID: 33470671]
Level 3 (low-level) evidence
[43]
Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma--evaluation of the retinal nerve fiber layer and visual field. American journal of ophthalmology. 2014 Nov:158(5):905-15. doi: 10.1016/j.ajo.2014.07.030. Epub 2014 Jul 25
[PubMed PMID: 25068638]
[44]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27
[PubMed PMID: 34501306]
[45]
Magan T, Tanner A, Fajardo-Sanchez J, Lim KS, Goyal S, Rodrigues I, Amaya L, Trikha S, Kulkarni A, Hammond C, Lascaratos G, Yu-Wai-Man C. Long-term outcomes in Primary congenital glaucoma, aniridia and anterior segment dysgenesis. European journal of ophthalmology. 2022 Sep:32(5):2920-2927. doi: 10.1177/11206721211073208. Epub 2022 Jan 10
[PubMed PMID: 35001688]
[46]
Janssens R, van Rijn LJ, Eggink CA, Jansonius NM, Janssen SF. Ultrasound biomicroscopy of the anterior segment in patients with primary congenital glaucoma: a review of the literature. Acta ophthalmologica. 2022 Sep:100(6):605-613. doi: 10.1111/aos.15082. Epub 2021 Dec 22
[PubMed PMID: 34939345]
[47]
Moore DB, Ben Zion I, Neely DE, Roberts GJ, Sprunger DT, Plager DA. Refractive outcomes with secondary intraocular lens implantation in children. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2009 Dec:13(6):551-4. doi: 10.1016/j.jaapos.2009.09.012. Epub
[PubMed PMID: 20006814]
[48]
Sihota R, Selvan H, Sharma A, Gupta N, Shakrawal J, Angmo D, Dada T, Upadhyay A. Severity of visual field defects in primary congenital glaucoma and their risk factors. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020 Jul:258(7):1483-1491. doi: 10.1007/s00417-020-04677-w. Epub 2020 Apr 15
[PubMed PMID: 32296990]
[49]
Naik A, Sihota R, Mahalingam K, Angmo D, Dada T, Kumar A, Kumar A, Gupta A. Evaluation of visual field changes with retinal nerve fiber layer thickness in primary congenital glaucoma. Indian journal of ophthalmology. 2022 Oct:70(10):3556-3561. doi: 10.4103/ijo.IJO_396_22. Epub
[PubMed PMID: 36190046]
[50]
Abdeen W, Esmael AF, Gawdat G, El-Fayoumi D. Anterior chamber angle features in primary congenital glaucoma infants using hand-held anterior segment-oct. Eye (London, England). 2022 Jun:36(6):1238-1245. doi: 10.1038/s41433-021-01583-1. Epub 2021 Jun 11
[PubMed PMID: 34117386]
[51]
Makhoul NJ, Wehbi Z, El Hadi D, Noureddine B, Boustany RM, Al-Haddad C. Whole-exome screening for primary congenital glaucoma in Lebanon. Ophthalmic genetics. 2023 Jun:44(3):234-245. doi: 10.1080/13816810.2023.2189949. Epub 2023 Mar 30
[PubMed PMID: 36995002]
[52]
Gusson E, Chemello F, Longo R, Franzolin E, Vesentini R, Verlato G, Marchini G. Primary congenital glaucoma surgery: outcomes and visual function. International ophthalmology. 2021 Nov:41(11):3861-3867. doi: 10.1007/s10792-021-01957-0. Epub 2021 Jul 23
[PubMed PMID: 34297306]
[53]
Strzalkowska A, Strzalkowski P, Stingl JV, Pfeiffer N, Schuster AK, Hoffmann EM. Influence of different primary surgical techniques on long-term intraocular pressure and medication in glaucoma after congenital cataract surgery. PloS one. 2023:18(7):e0286318. doi: 10.1371/journal.pone.0286318. Epub 2023 Jul 5
[PubMed PMID: 37406023]
[54]
Geyer O, Segal A, Melamud A, Wolf A. Clinical Outcomes After Ahmed Glaucoma Valve Implantation for Pediatric Glaucoma After Congenital Cataract Surgery. Journal of glaucoma. 2021 Jan 1:30(1):78-82. doi: 10.1097/IJG.0000000000001689. Epub
[PubMed PMID: 33003112]
Level 2 (mid-level) evidence
[55]
Samant M, Medsinge A, Nischal KK. Pediatric Glaucoma: Pharmacotherapeutic Options. Paediatric drugs. 2016 Jun:18(3):209-19. doi: 10.1007/s40272-016-0174-4. Epub
[PubMed PMID: 27093864]
[56]
Lewis RA, Christie WC, Day DG, Craven ER, Walters T, Bejanian M, Lee SS, Goodkin ML, Zhang J, Whitcup SM, Robinson MR, Bimatoprost SR Study Group. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. American journal of ophthalmology. 2017 Mar:175():137-147. doi: 10.1016/j.ajo.2016.11.020. Epub 2016 Dec 22
[PubMed PMID: 28012819]
Level 1 (high-level) evidence
[57]
Ghate D, Wang X. Surgical interventions for primary congenital glaucoma. The Cochrane database of systematic reviews. 2015 Jan 30:1():CD008213. doi: 10.1002/14651858.CD008213.pub2. Epub 2015 Jan 30
[PubMed PMID: 25636153]
Level 1 (high-level) evidence
[58]
Broughton WL, Parks MM. An analysis of treatment of congenital glaucoma by goniotomy. American journal of ophthalmology. 1981 May:91(5):566-72
[PubMed PMID: 7234937]
[59]
SMITH R. A new technique for opening the canal of Schlemm. Preliminary report. The British journal of ophthalmology. 1960 Jun:44(6):370-3
[PubMed PMID: 13832124]
[60]
Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, Vela MA, Lynn MJ. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2000 Aug:4(4):205-10
[PubMed PMID: 10951295]
[61]
McPherson SD Jr, Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. American journal of ophthalmology. 1983 Apr:95(4):427-31
[PubMed PMID: 6837685]
[62]
Cairns JE. Trabeculectomy. Preliminary report of a new method. American journal of ophthalmology. 1968 Oct:66(4):673-9
[PubMed PMID: 4891876]
[63]
Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology. 2000 Mar:107(3):422-9
[PubMed PMID: 10711876]
[64]
Elhofi A, Helaly HA. Non-Penetrating Deep Sclerectomy versus Trabeculectomy in Primary Congenital Glaucoma. Clinical ophthalmology (Auckland, N.Z.). 2020:14():1277-1285. doi: 10.2147/OPTH.S253689. Epub 2020 May 12
[PubMed PMID: 32494118]
[65]
Razeghinejad MR, Kaffashan S, Nowroozzadeh MH. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2014 Dec:18(6):590-5. doi: 10.1016/j.jaapos.2014.08.008. Epub 2014 Nov 12
[PubMed PMID: 25459201]
[66]
Malek I, Sayadi J, Choura R, Mekni M, Rayhane H, Khairallah M, Nacef L. Long-Term Results of Combined Trabeculotomy Trabeculectomy in Primary Congenital Glaucoma. Journal of glaucoma. 2023 Oct 1:32(10):848-853. doi: 10.1097/IJG.0000000000002229. Epub 2023 Apr 10
[PubMed PMID: 37079484]
[67]
Yazdani S, Pakravan M, Gerami E, Doozandeh A, Esfandiari H, Sharifipour F. Trabeculotomy Versus Combined Trabeculotomy-Trabeculectomy for Management of Primary Congenital Glaucoma. Journal of glaucoma. 2022 May 1:31(5):346-350. doi: 10.1097/IJG.0000000000001981. Epub 2022 Jan 10
[PubMed PMID: 34999664]
[68]
al Faran MF, Tomey KF, al Mutlaq FA. Cyclocryotherapy in selected cases of congenital glaucoma. Ophthalmic surgery. 1990 Nov:21(11):794-8
[PubMed PMID: 2270165]
Level 3 (low-level) evidence
[69]
Elhefney EM, Mokbel TH, Hagras SM, AlNagdy AA, Ellayeh AA, Mohsen TA, Gaafar WM. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. European journal of ophthalmology. 2020 Sep:30(5):1149-1155. doi: 10.1177/1120672119858226. Epub 2019 Jul 1
[PubMed PMID: 31256680]
[70]
Gouider D, Choura R, Mekni M, Sayadi J, Malek I, Nacef L. Sclerocornea: A rare ocular condition. Journal francais d'ophtalmologie. 2021 Sep:44(7):1089-1091. doi: 10.1016/j.jfo.2021.05.001. Epub 2021 Jun 18
[PubMed PMID: 34148700]
[71]
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub
[PubMed PMID: 33913840]
Level 2 (mid-level) evidence
[72]
Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. European journal of ophthalmology. 2022 Sep:32(5):NP87-NP91. doi: 10.1177/11206721211006564. Epub 2021 Mar 28
[PubMed PMID: 33779337]
Level 3 (low-level) evidence
[74]
Raven ML, Rodriguez ME, Potter HD. Corneal Leukoma with Features of Both Sclerocornea and Peter's Anomaly. Ophthalmology. 2016 Sep:123(9):1988. doi: 10.1016/j.ophtha.2016.05.011. Epub
[PubMed PMID: 27549880]
[75]
Altamirano F, Ortiz-Morales G, O'Connor-Cordova MA, Sancén-Herrera JP, Zavala J, Valdez-Garcia JE. Fuchs endothelial corneal dystrophy: an updated review. International ophthalmology. 2024 Feb 12:44(1):61. doi: 10.1007/s10792-024-02994-1. Epub 2024 Feb 12
[PubMed PMID: 38345780]
[77]
Balamurugan S, Gurnani B, Kaur K, Gireesh P, Narayana S. Traumatic intralenticular abscess-What is so different? The Indian journal of radiology & imaging. 2020 Jan-Mar:30(1):92-94. doi: 10.4103/ijri.IJRI_369_19. Epub 2020 Mar 30
[PubMed PMID: 32476758]
[78]
Kaur K, Gurnani B, Devy N. Atypical optic neuritis - a case with a new surprise every visit. GMS ophthalmology cases. 2020:10():Doc11. doi: 10.3205/oc000138. Epub 2020 Feb 27
[PubMed PMID: 32269909]
Level 3 (low-level) evidence
[79]
de Silva DJ, Khaw PT, Brookes JL. Long-term outcome of primary congenital glaucoma. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2011 Apr:15(2):148-52. doi: 10.1016/j.jaapos.2010.11.025. Epub
[PubMed PMID: 21596293]
[80]
Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Transactions of the American Ophthalmological Society. 1982:80():321-5
[PubMed PMID: 7182965]
[81]
Kaur K, Kannusamy V, Mouttapa F, Gurnani B, Venkatesh R, Khadia A. To assess the accuracy of Plusoptix S12-C photoscreener in detecting amblyogenic risk factors in children aged 6 months to 6 years in remote areas of South India. Indian journal of ophthalmology. 2020 Oct:68(10):2186-2189. doi: 10.4103/ijo.IJO_2046_19. Epub
[PubMed PMID: 32971637]
[82]
Christy J, Jain N, Gurnani B, Kaur K. Twinkling Eye -A Rare Presentation in Neovascular Glaucoma. Journal of glaucoma. 2019 May 23:():. doi: 10.1097/IJG.0000000000001287. Epub 2019 May 23
[PubMed PMID: 31135586]
[83]
Gurnani B, Kaur K, Sekaran S. First case of coloboma, lens neovascularization, traumatic cataract, and retinal detachment in a young Asian female. Clinical case reports. 2021 Sep:9(9):e04743. doi: 10.1002/ccr3.4743. Epub 2021 Aug 30
[PubMed PMID: 34484773]
Level 3 (low-level) evidence
[84]
Gurnani B, Kaur K, Gireesh P. A rare presentation of anterior dislocation of calcified capsular bag in a spontaneously absorbed cataractous eye. Oman journal of ophthalmology. 2021 May-Aug:14(2):120-121. doi: 10.4103/ojo.OJO_65_2019. Epub 2021 Jun 28
[PubMed PMID: 34345149]
[85]
Gurnani B, Kaur K, Gireesh P. Rare Coexistence of Bilateral Congenital Sutural and Cortical Blue Dot Cataracts. Journal of pediatric ophthalmology and strabismus. 2020 Jan 1:57(1):68. doi: 10.3928/01913913-20191011-01. Epub
[PubMed PMID: 31972045]
[86]
Kaur K, Gurnani B, Kannusamy V, Yadalla D. A tale of orbital cellulitis and retinopathy of prematurity in an infant: First case report. European journal of ophthalmology. 2022 Nov:32(6):NP20-NP23. doi: 10.1177/11206721211026098. Epub 2021 Jun 17
[PubMed PMID: 34137305]
Level 3 (low-level) evidence