Introduction
Wilhelm Röentgen, in 1895 used the mathematical "X" to describe unknown rays, which he discovered, and two weeks after his discovery, he produced the first X-ray image of his wife’s hand, the first medical imaging photo published in December 1895.[1] The New York Times then predicted the “transformation of modern surgery by enabling the surgeon to detect the presence of foreign bodies.” Since his discovery, X-rays have advanced from fuzzy images of bones and foreign bodies to 3D cone beam imaging within a century since his discovery.
X-rays are the most common evaluation tool utilized in orthopedics to diagnose and evaluate various musculoskeletal diseases. Mobile X-ray units developed by Madam Marie Curie were the first to run to help military surgeons on the field. While radiography provides static images, fluoroscopy uses X-rays to obtain real-time images and videos of the internal structure and function of the patient. The first fluoroscope consisted of an X-ray tube and a fluorescent screen, but the major problem was producing images with sufficient brightness for visualization. Image intensifiers using optical lenses and mirrors for magnification were developed, and later a video camera and monitor were attached to allow better viewing.
The C-arm, an X-ray machine with a half-moon frame, was developed, making the X-ray machine mobile in all directions.[2] The image intensifier with a monitor connected to the C-arm, a work-station unit, enhanced picture quality, and ease of viewing. The application of digital imaging and processing techniques improved image quality and digital recording of the images. The fluoroscopy imaging unit is one of the most valuable tools in the armamentarium of an orthopedic surgeon. Although utilized daily, there is a paucity of knowledge regarding proper usage and safety.
This article outlines basic fluoroscopy protocols to enable young surgeons to effectively and safely use radiation to their advantage.
Technique or Treatment
Obtaining optimum diagnostic quality image is critical while decreasing patient and surgeon dosage during the procedure. We outline some techniques to limit the radiation exposure and scatter, and at the same time, obtain a good image.
The primary beam is the main source of radiation to the patient, whereas the scatter is the source of exposure to the surgeon and operator. Fluoroscopy systems contain variables that regulate the amount of radiation and adjust image quality. For example, the tube voltage is adjusted in kilovolts (kV), the tube current in milliampere, and the pulse duration in milliseconds. A higher kilovolt setting will allow lower current (milliampere) delivery and hence lower radiation exposure. Hence, the equipment includes a variety of options for specific procedures.
The intensifier must be as close to the patient as feasible. This prevents the beam from dispersing and reduces the radiation required for a sharp image. Removal of the anti-scatter grid decreases the total radiation dose but with a slight increase in the amount of radiation scatter. A balance must be attained between the total dosage and the amount of scatter. Pulsed fluoroscopy reduces fluoroscopy time by 76% and radiation dose by 64% compared with continuous fluoroscopy. Decreasing the frame rate further decreases the effective radiation. Collimation must be used whenever feasible.[9] A reduced field of view (FOV) decreases scatter and decreases the exposure of the patient to unwanted parts.
Magnification increases the radiation dose as well as the scatter. The need for magnification is limited by digital zooming and the use of large displays. An increase of scattered radiation is seen when the gantry position is more than 30 degrees in left or right anterior oblique angulations or 15 degrees in cranial angulation. Additional exposure can be due to incorrect coordination between radiographers and surgeons due to non-standard means of communication. Effective methods of communication with accurate terminology must be developed between the surgical team and C-arm technicians.[10]
Joint Injections Under Fluoroscopic Guidance
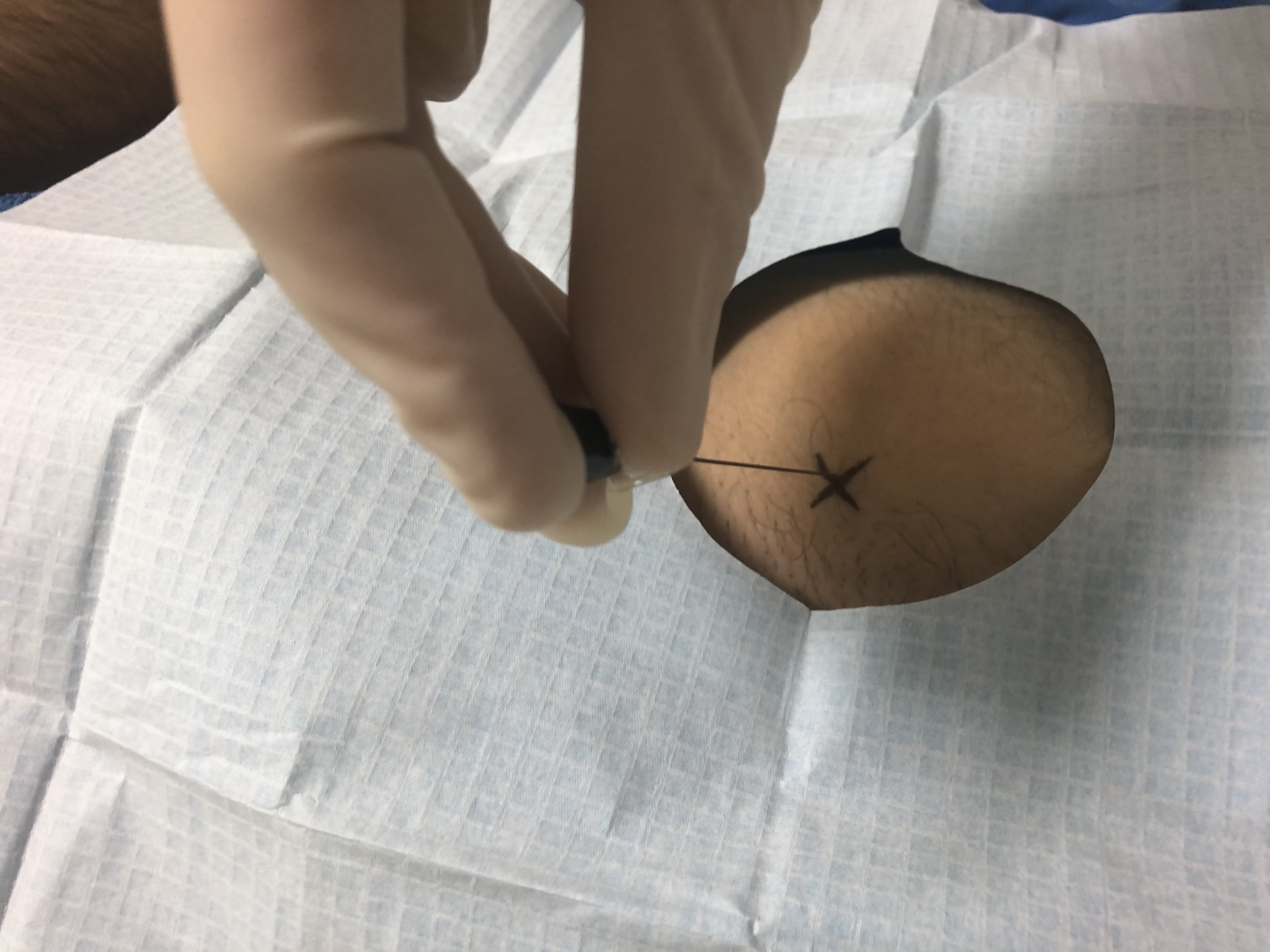
All joint injections are carried out under strict aseptic precautions and require appropriate draping and preparation. Proper patient positioning before the procedure ensures safe and efficient access to the joint as well as patient comfort. A radio-opaque object is placed on the skin over the target joint to mark the skin entry. Sterile preparation is performed. Local anesthetic is injected into the overlying skin and tract (e.g., 2% lidocaine). The appropriate length and gauge needle is chosen and is advanced into the joint with intermittent fluoroscopic guidance.[11]
Imaging provides a visual reference, but it is necessary to develop a tactile sense of the needle encountering different tissues. The intra-articular position of the needle is confirmed either by air arthrogram, which shows an intra-articular air ring, or by saline distension of the joint. Needle placement is further confirmed when the contrast medium can be injected with little resistance. It flows into the joint recesses rather than clustering around the needle tip if the needle is intra-articular. For diagnostic injections, an iodinated contrast medium is used. Therapeutic injections include steroids such as triamcinolone, hyaluronic acid, platelet-rich plasma, etc.
A summary of commonly used approaches to joint injections is given below:
Considerations in Individual Joints
Shoulder
The shoulder joint can be approached anteriorly or posteriorly. Anterior approaches are the most commonly used shoulder approaches.[12] Anteriorly the Schneider approach and rotator interval approach can be used. The patient is supine, and the shoulder is positioned in an anteroposterior view. The arm is held in external rotation with a weight in hand.
In the rotator interval approach, the target is the upper medial quadrant of the humeral head. The coracoid process is avoided in this position. The needle enters the space between the supraspinatus and subscapularis muscles. The external rotation draws the biceps tendon out of the needle path. It is a shorter distance from the skin and avoids damage to other structures. An unintentional bursal injection is a common problem with this method.[13]
In the Schneider approach, the needle enters the junction of the middle and inferior thirds of the medial humeral head. This may be painful as the needle enters through the subscapularis muscle and risks damaging the labrum. A 22-gauge needle is inserted until it contacts the bone and is slightly withdrawn. The flow of contrast medium and opacification of the joint space and the subcoracoid recess confirm intra-articular positioning. For the posterior approach, the patient can be placed either in a prone position with the arm in a neutral position or a sitting position. In the prone position, the shoulder is elevated with a triangular foam pad placed under the torso. The needle is advanced into the glenohumeral joint through the infraspinatus between the free edge of the posterior labrum and the hypoechoic articular cartilage of the posterior humeral head. With this technique, injury to neurovascular bundles, the suprascapular nerve, and the circumflex scapular vessels may occur if the needle is placed too medially.[14]
Elbow
The patient is either prone with arms hanging or seated with the arm in 90-90 over a radiolucent table.[15] The hand is supinated with the thumb up to open the joint. The radiocapitellar joint is positioned parallel to the beam. For the trans-triceps approach, the elbow is flexed, and a point is marked immediately above and lateral to the olecranon. The needle is advanced until it comes into contact with the olecranon fossa of the humerus. The advantage of this approach is that it decreases the likelihood of contrast leakage in the lateral aspect of the joint, thus avoiding a diagnostic dilemma with subsequent CT or MRI, especially when injury to the lateral elbow soft tissue is suspected.
Wrist
Injection into the radiocarpal joint is performed to evaluate the triangular fibrocartilage complex and intrinsic ligaments for complete tears. The patient lies prone on the table or sits on a stool with the palm facing down on the table. A dorsal approach is used for all wrist compartments. To improve the profile, a soft towel is placed beneath the wrist to maintain passive wrist flexion.[16] Osseous overlap can be corrected by a slight craniocaudal tilt of the fluoroscope. Ulnar deviation opens the joint on the radial side, the more common site of injection. For radial-side injection, the needle is advanced to the proximal cortex of the scaphoid, ulnar to the tubercle. An ulnar-side injection of the radiocarpal joint is performed by directing the needle to the proximal edge of the triquetrum, adjacent to the pisiform. For injection into the distal radioulnar joint, the radial margin of the distal ulna is targeted just proximal to the physeal scar.
The mid-carpal compartment injection is done at the confluence of the triquetrum, lunate, capitate, and hamate. This space may communicate with the common carpometacarpal joint but not with the radio-carpal joint.
Hip
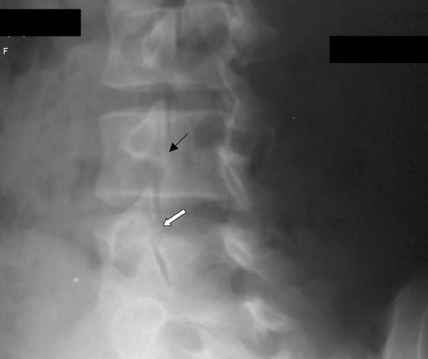
The hip capsule must be pierced on the anterolateral surface of the femoral head or neck between the acetabular rim and the intertrochanteric line. The patient is supine, and the lower extremity is internally rotated 10 to 15 degrees.[17] To avoid the neurovascular bundle and iliopsoas tendon, the lateral aspect of the femoral head-neck junction is targeted. The tip of the greater trochanter and the anterior superior iliac spine (ASIS) is marked, and a line is drawn between them. At the junction between the upper third and lower two-thirds at the anterior border of the gluteus medius is the needle entry point with a posterior tilt of 30 degrees. The needle is inserted till it touches the bone; if it does not touch the bone, the needle is reinserted with a lesser angle. The needle placement is confirmed by air arthrogram or saline injection.
Fluoroscopic Arthrography in DDH
Hip arthrography is a dynamic study of the hip joint. It is performed by injecting a radiopaque dye into the hip joint and carrying out a fluoroscopic examination with the patient under anesthesia and is usually performed in conjunction with a closed reduction. Arthrography helps in determining the underlying cartilaginous profile and also the dynamic stability of the hip. Arthrography performed in combination with a closed reduction can assess the adequacy of the reduction. An increased medial joint space, demonstrated by medial pooling of the dye and a rounded or interposing limbus, is indicative of poor results.[18]
Fluoroscopy in Trauma
The anatomical reconstruction of joint surfaces, limb alignment, and rotation orientation are crucial in fracture fixation to achieve optimum function and range of motion. With continuing advancements in implants and surgical techniques, high-resolution intra-operative imaging has become imperative in trauma surgery. Without the real-time visualization of the anatomy and the position of implants in relation to the bone and evaluation of the reconstruction of joint surfaces and bony alignment, the rapid evolution of minimally invasive surgery would not have been possible. Intra-operative fluoroscopy aids in continuously controlling these parameters while doing fracture fixation.[19] For example, the percutaneous placement of pedicle screws in spine surgery, tunneling of implants in the tibial or femoral metaphyseal fractures with specially designed plates, calcaneus osteosyntheses with slit-in plates, and closed reduction and intramedullary nailing of femur/tibia shaft fractures, require intra-operative fluoroscopy guidance.[20]
Even with complete access to the surgical view in conventional total hip arthroplasty, intra-operative images are performed to confirm the acetabular cup's alignment and orientation and rule out fractures of the acetabulum or the femoral shaft. Standards for axis alignments and patient positioning exist in radiology departments, but the intra-operative settings vary due to the positioning requirement of the surgical procedure as well as patient-related factors such as obesity or limitations in joint movement.[21][22] The surgeon must ensure the correct position of the operational field and acquire the standard views of the region with the C-arm.[23][24] Knowledge of standard position on the operating table ensures decreased exposure and precise imaging.
The Distal Radius
The joint surface is tilted volar by 5 to 10 degrees in the AP view.[24] When controlling the volar plate position and reduction, the elbow has to be elevated to the extent of 5 degrees to 10 degrees to balance out this anatomical tilt. It is possible to accurately assess reduction, implant positioning, and screw placement in this position. In the lateral view, where the joint line is tilted 23 to 30 degrees, the hand is similarly elevated to this level for proper joint visualization.
The Proximal Humerus
The patient is positioned in the beach-chair position in osteosynthesis of the proximal humerus. Hence, the upper arm is already tilted towards the floor. This is evened out by tilting the C-arm so that the beam is perpendicular to the humeral shaft axis. The standard views are defined as an AP view with a maximum extension of the greater tubercle and a lateral view with 90 degrees external rotation of the humerus. The spherical shape of the humeral head must be considered when assessing screw length. Dynamic fluoroscopy while rotating the arm might be helpful to assess the screw length.
The Proximal Femur
A traction table is usually used for fracture reduction, and the contralateral leg is elevated in a leg holder.[21] The C-arm is placed perpendicular to the floor for an ap view and obliquely between the legs for an axial view. When adjusting the axial view, the anteversion of the femoral neck of 10 to 15 degrees is compensated by tilting the C-arm.
The Ankle Joint
While positioning the C-arm for osteosynthesis of the ankle joint, the hazard of collision of the device with the patient is less. The lower leg is internally rotated by 15 to 20 degrees to obtain the Mortise view. In this view, the medial and lateral joint space should be equal, and no double contour of the margins of the talus should be seen. For the lateral view, the condyles of the talus must be exactly above each other, and the tibiotalar joint space must be completely visible.[25]
Spinal Fluoroscopy- Facet Joint Injections
Facet joints are one of the most common causes of low back pain (LBP). Hence, facet joint interventions like intraarticular steroid injections and medial branch blocks are commonly performed.
Anatomical Considerations
Synovial facet joints are simple in the cervical and thoracic regions but very complex in the lumbar region. Engel and Bogduk described three types of lumbar intracapsular structures. The first structure includes adipose tissue pads covered by the synovial membrane. The second is fibro-adipose meniscoid tissue which is also covered by the synovial membrane and projects out from the joint capsule at the superior and inferior poles. The third is the connective tissue rims located along the anterior and posterior margins and are folds of the fibrous layer of the joint capsule. Each lumbar facet joint has space that can accommodate 1 to 1.5 ml of fluid.[26]
Indications For Facet Joint Injections
- Average pain levels >5 on a scale of 0 to 10
- Intermittent or continuous LBP causing functional disability
- Somatic or non-radicular LBP and lower extremity pain lasting at least three months
- Failed conservative treatment
- Lack of evidence of either discogenic or sacroiliac joint pain or radiculitis
- Contraindications or inability to undergo physical therapy or inability to tolerate oral medications.
The Technique of Localization of Facet Joint Under the Fluoroscope
The fluoroscopic-guided technique of lumbar facet injection is considered the most reliable. The patient is placed prone on the procedure table with the fluoroscope over the target level. A pillow is placed under the abdomen to reduce lumbar curvature, allowing easier entry into the joint. It also allows for the patient's head and face to be supported comfortably. A baseline anteroposterior (AP) fluoroscopic view of the lumbar spine should be obtained, and the C-arm is oriented. The patient is turned slowly so that the side to be injected is off the table till the facet joint is seen in profile and the position is secured. Alternatively, the tube may be tilted. The posterior aspect of the facet joint is further from the midline than the anterior.[27]
During posteroanterior(PA) fluoroscopy, first, the posterior portion of the joint is seen. Over-rotation has to be avoided as it brings the anterior portion of the joint into the profile. This makes needle placement into the joint impossible. The upper lumbar spine requires an obliquity of 30 degrees, while the lower lumbar spine requires up to 60 degrees obliquity. Once localized, the needle is directed vertically into the center of the joint space. Alternatively, the inferior recess is targeted since it lies posteriorly, has no direct neural relations, and is relatively capacious, hence easy to enter.
Clinical Significance
Fluoroscopy in Pregnancy
Trauma affects up to 8% of pregnancies; the cause varies from motor vehicle accidents to self-fall and is one of the leading causes of maternal morbidity and mortality. The National Council on Radiation Protection and Measurements and the International Commission on Radiological Protection have set a recommended maximum exposure of a fetus to radiation at 5 cGy. At this level of exposure, the assumed risk to the developing embryo is less than the spontaneous risks present during the development of any human fetus. It is observed that exposure of the maternal thyroid gland to diagnostic radiation was associated with a slight decrease in birth weight.[28]
Guidelines for imaging in pregnancy:
- Women must be counseled that X-ray exposure from a single diagnostic procedure does not result in harmful fetal effects.
- Specifically, exposure to less than 5 rad is not associated with increased fetal anomalies or pregnancy loss.
- Concern about high-dose ionizing radiation exposure should not prevent medically indicated diagnostic procedures from being performed in pregnancy.
- Imaging procedures not associated with ionizing radiation should be considered instead of X-rays when appropriate.
- The use of radioactive isotopes of iodine is contraindicated during pregnancy.
- Radiopaque and paramagnetic contrast agents are unlikely to cause harm and may be of diagnostic benefit.
Recent Advances
The continuing advances in imaging have resulted in a variety of components like image intensifiers which automatically adjust the sharpness and contrast of an image irrespective of the exposure, view of the latest image to provide a continuum in pulsed fluoroscopy, and real-time radiation dose display to keep the surgeon aware of the exposure.[29]
Flat-panel detectors are a revolution in medical imaging as they provide high image quality and dose reduction compared with a standard C-arm. Mobile flat-panel C-arms reduce intra-operative radiation dose, have an increased field of view of images due to a larger detector diameter, and give better soft-tissue resolution. Compared with standard C-arms, the source to image distance increases, and the surgeon has a larger workspace with decreased scatter.
Intra-operative three-dimensional (3D) imaging can ensure adequate fracture reduction in situations where the joint surface is not completely visible in 2D imaging, especially in joints having a concave configuration, such as the acetabulum or in joints irregularly shaped bones like the calcaneus. Cone-beam CT is a 3D data set that can be used with a flat-panel detector. It acquires a large number of images that are converted to 3D images by computational methods.[30] Intra-operative 3D imaging with mobile C-arms is an important adjunct in the reconstruction of complex joint fractures. Control of fracture reduction and implant positioning with high image quality reduces the need for secondary revisions in trauma surgeries.[31] O-arm, the intraoperative navigation system significantly reduces the malposition rate of the pedicle screws and improves ilio-sacral screw placement.
Nursing, Allied Health, and Interprofessional Team Interventions
Team Safety Protocol
Natural radiation exposure up to a dose of 2 to 200 mSv per year is presently based on the geographical area.[32] The average dose due to radiation exposure from medical devices is about 2 mSv. The maximum permissible dose (MPD) is the upper limit of the radiation dose that one can receive without the risk of significant side effects. The annual whole-body dose limit for physicians is 50 mSv. C-arms pose a potential threat to the patient as well as to the staff in the operating room. As low as reasonably achievable (ALARA) suggests that even a small dose of diagnostic radiation must be avoided if it has no direct benefit in the procedure.[33] This is achieved by using three basic protective measures in radiation safety: time, distance, and shielding.
The surgeons have to weigh the hazard to benefit ratio due to the radiation and monitor the individual dose.[34] The use of a personal dosimeter within the radiation control zone of 4 meters is compulsory in many countries. Lead gowns with thyroid protection must be worn by all staff in the operating room. The lead gowns with thyroid protection need to be checked regularly to ensure intact material. The patient must be protected with lead mats as long as they do not hinder the surgical field. The surgeon's knowledge of the settings of the device and its application decreases the dosage of exposed radiation. Pulsed fluoroscopy and single images are commonly used instead of continuous fluoroscopy as it decreases the radiation. Dynamic fluoroscopy should be used only if necessary and for a very short duration.[34]
The surgeon needs to keep his hands out of the beam effectively. By making the field of view smaller and using a split diaphragm, scattered radiation can be reduced, thereby decreasing the exposure to the surgeon's hand. Distance is the most effective method to reduce exposure as exposure is decreased exponentially with distance. For all personnel with the risk of regular exposure to ionizing radiation, the use of dosimeters must be made mandatory. Real-time dosimeters allow for direct visualization of the radiation dose, which will help increase awareness regarding radiation hazards in personnel.