Continuing Education Activity
Galactoceles are benign milk retention cysts that primarily arise in lactating or recently lactating women due to a persistent obstruction of the lactiferous duct. This obstruction causes milk to accumulate in a cyst-like structure. Typically presenting as a moderately firm, painless mass, galactoceles may gradually or rapidly increase in size, with fluctuations noted throughout the day. While they are generally not associated with pain or inflammation, complications can lead to infection.
Ultrasonography typically reveals a cystic fluid collection, and in symptomatic cases, aspiration may be performed for both diagnostic and therapeutic purposes. Although many galactoceles resolve spontaneously, those that are symptomatic or infected often require medical intervention, including drainage and antibiotics. This activity for healthcare professionals is designed to enhance the learner's competence in recognizing the clinical features of a galactocele, performing the recommended evaluation, and implementing an appropriate interprofessional management approach to improve patient outcomes.
Objectives:
Apply the pathophysiology leading to galactocele formation during the evaluation of a breast mass.
Identify the clinical features of a galactocele.
Select the indicated management approach for a galactocele.
Apply interprofessional team strategies to improve care coordination and outcomes in a patient with a galactocele.
Introduction
Galactoceles, occasionally termed lactocele or a lacteal cyst, are benign milk retention cysts that primarily arise in lactating or recently lactating patients due to a persistent obstruction of the lactiferous duct.[1] The term galactocele is derived from the Greek words galatea, meaning milky white, and cele, meaning pouch, and is the most commonly diagnosed benign breast mass in lactating patients.[2][1] This obstruction of the lactiferous duct causes milk to accumulate in a cystic structure, which can present clinically as a mass of varying size, from a barely palpable mass to a large mass measuring greater than 10 cm.[3] Typically presenting as a moderately firm, painless unilateral or bilateral mass, galactoceles may grow or decrease in size during their clinical course. While galactoceles are generally not associated with pain or inflammation, complications can arise, leading to infection.
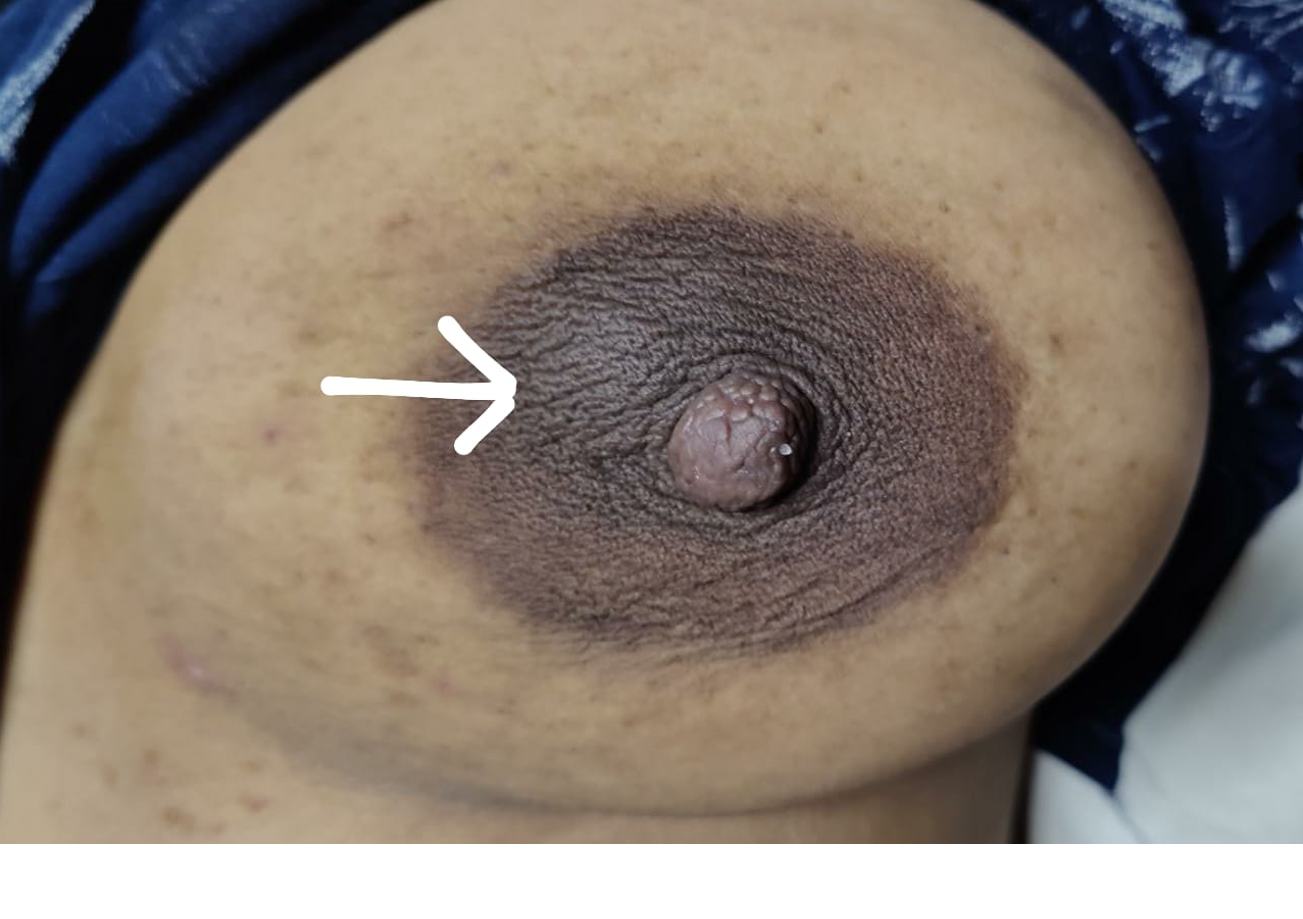
Although a galactocele can occur anywhere along the milk line extending from the axilla to the groin, it has a propensity to form in the retroareolar region of the breasts (see Image. Galactocele, Retroareolar Region of Left Breast). Differentiating galactoceles from other breast pathologies, such as cysts, fibroadenomas, abscesses, or malignancies, is essential. Ultrasonography of a galactocele typically reveals a cystic fluid collection, and in symptomatic cases, fine-needle aspiration (FNA) may be performed for both diagnostic and therapeutic purposes. Although many galactoceles resolve spontaneously, symptomatic or infected patients often require medical intervention, including drainage and antibiotics.[4]
Etiology
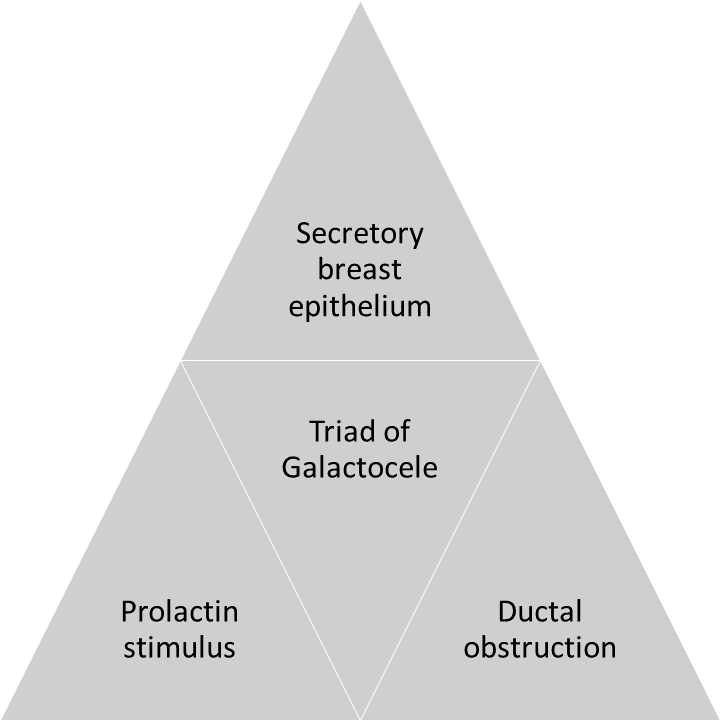
The triad of secretory breast epithelium, prolactin stimulus, and ductal obstruction are needed to develop a galactocele (see Image. Triad of Secretory Breas Epithelium).[5][6]
Secretory Breast Epithelium
Galactoceles most commonly present in patients after lactation cessation; galactoceles may also develop in the third trimester of pregnancy and during lactation. Ductal proliferation is predominantly controlled by estrogen, whereas acinar differentiation is a progesterone effect facilitated by estrogen. These hormones contribute to mammogenesis. The hormonal influence of chorionic gonadotropin forms lobules that have acini with epithelial cells of larger size and number. Small amounts of milk can be secreted as early as week 16 of gestation.[7] Factors that predispose to galactocele formation include:
- Difficulty breastfeeding (eg, infants with cleft palate)
- Infant conditions or settings in which breastfeeding is contraindicated and lactiferous ducts are not emptied [8]
- Phenylketonuria, rare amino acidurias, and classic galactosemia
- Untreated congenital diaphragmatic hernia, esophageal atresia, tracheoesophageal fistula, intestinal obstruction
- Risk of infant exposure to infectious pathogens via breast milk, such as HIV, human T-cell lymphotropic virus, or Ebola virus
- Risk of infant exposure to maternal medications or radioactive agents via breast milk
- Combined oral contraceptive use due to excessive stimulation of the breast epithelium [5]
Prolactin Stimulus
Approximately 32 cases of galactoceles have been reported in male infants due to transplacental passage of prolactin or a fetal pituitary adenoma causing chronic galactorrhea.[9][10][6] Rarely, galactocele can occur in adult men secondary to hyperprolactinemia.[11] Hyperprolactinemia may be secondary to a prolactinoma, which may be sporadic or associated with multiple endocrine neoplasia type 1 or hypogonadotropic hypogonadism.
Ductal Obstruction
In lactating patients, galactoceles develop due to a reduction in the diameter of lactiferous ductal openings, resulting in milk flow obstruction and accumulation of milk in a cystic cavity.[3] Recently, post–breast-augmentation galactoceles have also been reported; periareolar incisions are a significant risk factor for ductal injury and, subsequently, ductal obstruction. However, breast augmentation procedures performed via the inframammary approach, generally considered protective against the induction of postoperative galactorrhea, have been reported to cause galactocele in some cases.[12][13]
The proposed theory of postsurgical galactocele formation is surgical stimulation of the intercostal nerves leading to autonomic control over central neurogenic paths, diminishing dopamine output into the hypophyseal portal circulation, and increasing circulating prolactin levels and milk secretion.[13] Previous hormonal contraceptive use, pregnancy, and recent breastfeeding may also be contributing factors.[14] However, no genetic etiology or risk factors that increase galactocele formation have been identified.
Epidemiology
In general, benign breast diseases are seen in patients of all ages.[15] The incidence of breast cysts has been reported to increase between the ages of 45 and 50.[15] A palpable breast mass is one of the most common breast complaints in patients seeking medical evaluation.[16] Galactoceles are the most commonly diagnosed benign breast mass in lactating patients.[2][1]
The incidence of galactoceles in patients presenting with benign breast conditions in the nonhospital setting is estimated to be 4%. Additionally, galactoceles are reported in approximately 4% to 5% of breast imaging reporting and data system (BI-RADS) category 4 lesion biopsies.[17] However, the incidence may be higher as many galactoceles have mild symptoms and spontaneously resolve without treatment.
Pathophysiology
The primary pathophysiological mechanism of galactocele formation is mammary duct obstruction in the lactating breast. This obstruction may occur secondary to myriad factors, including incorrect breastfeeding techniques, decreased frequency of breastfeeding, trauma, inflammation, nipple abnormalities, or, rarely, an underlying tumor. Distal obstruction of the terminal duct lobular unit results in incomplete ductal emptying and milk accumulation, which causes proximal focal ductal dilatation and galactocele formation.[4][1] Sustained breast trauma, including mild trauma from aggressively squeezing the nipple to relieve a ductal obstruction or a "plugged duct," may precipitate an inflammatory reaction, which leads to galactocele formation.[3]
Transplacental passage of prolactin does not explain the rare development of galactocele in infant boys, who present with a new-onset breast swelling after a period of dormancy of a few months after birth. To explain this phenomenon, experts have hypothesized that neonates develop small retention cysts that remain dormant, with their secretory activity eventually ceasing over time.[18]
Histopathology
Histopathological examination of a galactocele typically reveals ducts lined by normal, flattened cuboidal secreting epithelium and myoepithelium of various sizes.[19][20] The presence of milk is confirmed chemically by a positive mucic acid test. Dilated anastomosing channels are lined by cuboidal epithelium, often with secretory activity. Sometimes, adjacent tissue may show evidence of pressure necrosis, foamy macrophages, and chronic inflammatory changes if cyst contents leak into adjacent tissues.[21][22] Cytologic evaluation of the cyst secretions frequently reveals necrotic cells, nuclear debris, mucins, lipids, lysozymes, albumin, and whey protein.[22]
History and Physical
Clinical History
Galactoceles most commonly form following breastfeeding cessation but may occur during the third trimester or active breastfeeding.[2] Additional historical risk factors for galactoceles include primiparity, difficult breastfeeding, aggressively trying to relieve ductal obstruction, or primarily using formula instead of breast milk due to an incomplete evacuation of milk in the lactiferous ducts.[3] Additionally, clinicians should obtain a medication history, specifically inquiring about galactagogues that increase the risk of galactocele formation, such as metoclopramide and domperidone.[23] Patients may report mild breast discomfort; severe symptoms, such as acute pain, erythema, or fever, are not observed unless the galactocele has become infected.[1]
Physical Examination
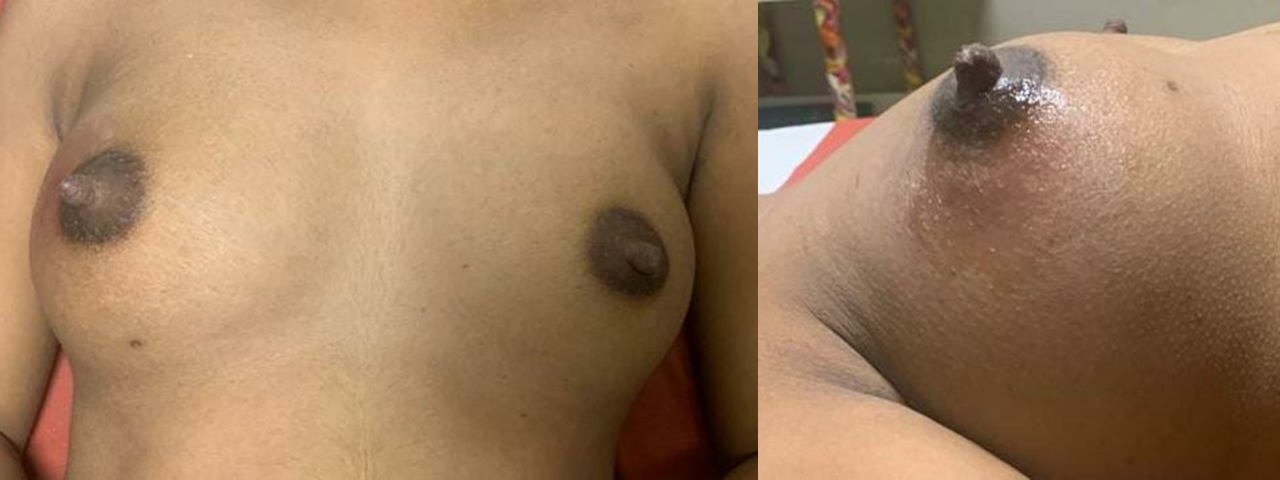
The physical examination of a patient with a galactocele will typically reveal a palpable retroareolar mass measuring 1 to 2 cm in diameter; galactoceles greater than 10 cm are uncommon but well-documented.[1] A gradual or rapid fluctuation in size is characteristic of galactoceles, and changes may occur throughout the day, with a brief decrease particularly observed following breastfeeding.[3] Galactoceles may be unilateral or bilateral and solitary or multiple (see Image. Galactocele).[1]
Evaluation
To avoid a missed diagnosis of a breast malignancy, which most commonly presents as a palpable mass, timely diagnostic imaging studies to evaluate these types of breast lesions are critical, even in pregnant and lactating patients.[24] Recommendations regarding the preferred imaging modality in lactating patients are limited; recent evidence-based guidelines have described an optimal approach.[25][20] Subsequently, any new palpable breast mass requires prompt investigation with a three-pronged assessment, including clinical examination, imaging, and aspiration with histologic assessment when needed for confirmation.[4]
Ultrasonography
Lactating patients typically have extremely dense breast tissue due to physiologic changes associated with pregnancy and lactation. Therefore, ultrasonography, which has a higher sensitivity and less radiation exposure than mammography, is the preferred imaging modality for these patients. The optimal imaging modality has historically been less well-defined for patients older than 30 who were pregnant or breastfeeding, for whom the American College of Radiology (ACR) Appropriateness Criteria had recommended mammography as the initial imaging modality.[20] Previously, the ACR recommended diagnostic ultrasonography as the initial imaging modality in any lactating or pregnant patient with a palpable breast mass, regardless of age.[24] This recommendation has remained the same for pregnant patients with a new palpable mass.[20]
However, current guidelines have changed for nonpregnant lactating individuals and now recommend that diagnostic imaging indications are the same in nonpregnant lactating and nonlactating patients. Therefore, the ACR now advises that diagnostic ultrasonography is initially indicated in lactating patients younger than 30 with a new palpable mass; in those older than 30, a diagnostic mammogram should be performed initially.[20]
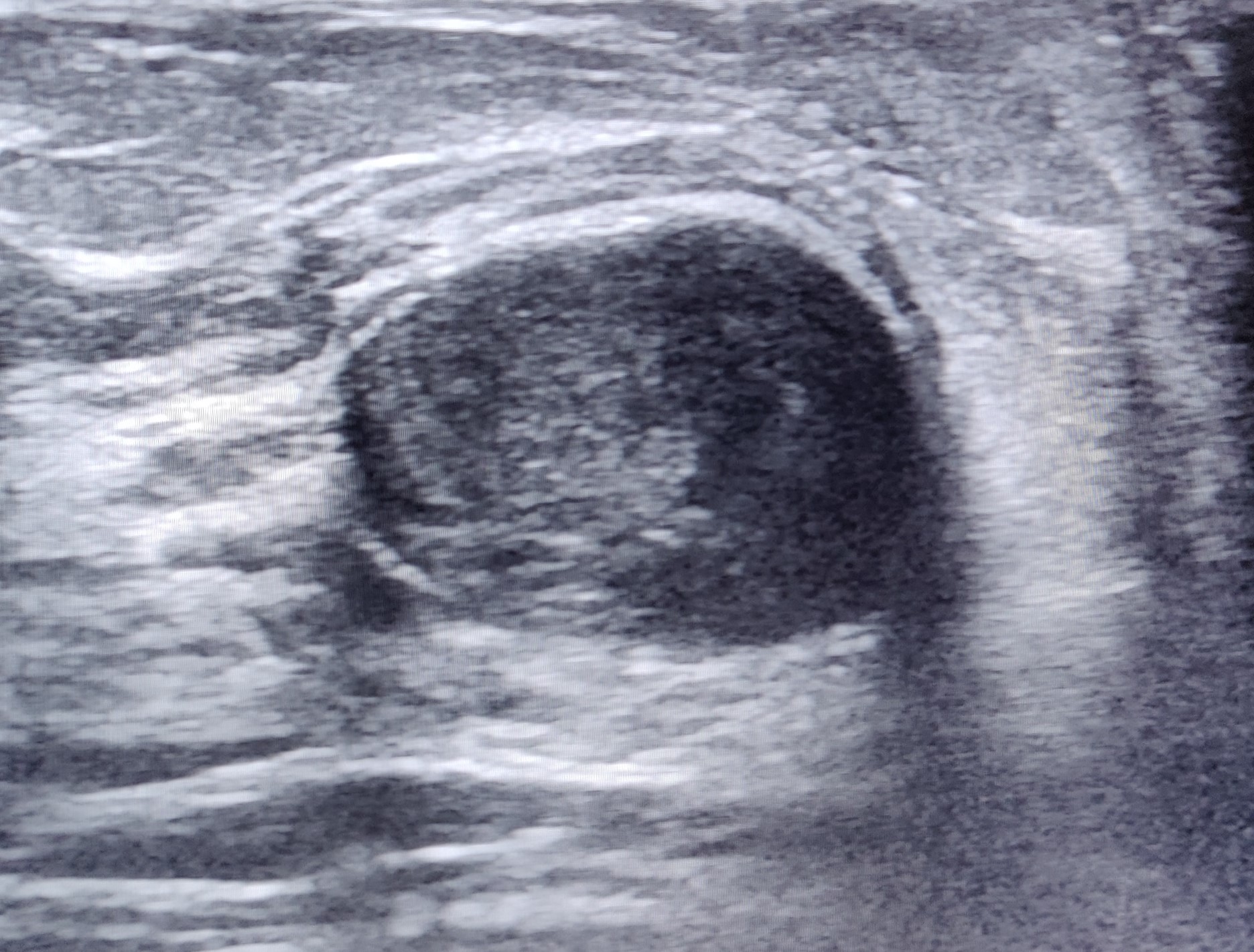
Ultrasonographic features of a galactocele can vary depending on the chronicity of the lesion. In acute cases, the internal contents of the galactocele are a fluid suspension, appearing more homogeneous with medium-level echoes. Conversely, the contents of a persistent galactocele are inspissated material; the appearance is heterogeneous, with internal fluid clefts and anechoic fluid rims (see Image. Inspissated Galactocele). Internal echogenic foci with acoustic shadowing are also secondary to milk products containing about 10% solids, fat, and desquamated epithelium. The distal acoustic enhancement is due to the fluid-filled cyst. The intensity of hypoechoic echo increases gradually due to the interface between the fat and water components.[7]
Ultrasonographic findings characteristic of galactocele include:
- Fat-fluid level, with the hypo- or hyperechogenic fat layer occupying the nondependent or upper part of the cyst
- Circumscribed oval or round shape
- Lack of internal vascularity on Doppler imaging
- Variable echogenicity: echotexture may be heterogeneous depending on fat and protein content
- Posterior acoustic enhancement is frequent, especially in uncomplicated galactoceles
- No internal calcifications; peripheral calcifications may occur in some cases but are generally absent internally [20][24]
Suspicious imaging features should be correlated clinically with signs such as redness, tenderness, and warmth.[26] Color Doppler investigation may be of some benefit in cases of galactocele, as internal vascularity will be absent.[27][19][27] Complex cysts should be carefully differentiated from breast carcinomas. Suspicious or atypical ultrasonographic features of a galactocele include:
- Indistinct or obscured margins
- Posterior acoustic shadowing, mimicking malignancy
- Internal heterogeneous echotexture, which may indicate atypical composition, especially if milk has thickened over time
- Absence of a clear fat-fluid level
- Internal vascularity
- Complex cystic or solid appearance; further investigation (eg, BI-RADS 4) may be warranted to rule out other pathologies [20][24]
Mammography
Diagnostic mammography is recommended as the initial imaging modality in lactating patients older than 30 to evaluate a new palpable breast mass or for further evaluation of a mass in lactating women younger than 30 with suspicious ultrasonographic features.[20] Mammogram findings consistent with a galactocele include:
- Fat-fluid level (seen best on a 90-degree lateral mammogram); characteristic fat layering above the fluid
- Well-circumscribed oval or round shape with smooth borders
- Peripheral rim calcifications [20]
Depending on the protein and fat content of the milk within the galactocele, lesions may have findings similar to other benign conditions, including lipoma, oil cyst, or pseudohamartomas; these findings may include a radiolucent mass or mixed densities.[20] Suspicious findings mammographic findings include:
- Indistinct or obscured margins
- Complex cystic or solid appearance
- Clustered or irregular microcalcifications
- Architectural distortion
- Absence of a pathognomonic fat-fluid level [20]
Aspiration may be performed for symptomatic relief or diagnostic confirmation, and in patients with galactoceles, it should yield milky fluid. A biopsy may be recommended to rule out malignancy in patients with infection or atypical imaging features, with BI-RADS categorization as appropriate.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) or diagnostic mammography may be performed for additional evaluation in lactating patients with dense breasts or as an initial evaluation in high-risk patients (eg, genetic mutation carriers and women who have received radiation therapy to the thorax or upper abdomen at an early age) aged 30 or older with a new palpable mass.[20] However, MRI is rarely needed for galactocele diagnosis, as ultrasonography is generally adequate for evaluation.[2] MRI will typically reveal a distinct fat-fluid level, which can be visualized by combining non–fat-saturated T1-weighted images (highlighting the fat component) and T2-weighted fat-saturated images (showing the fluid component). When positioned prone, the fat layer may shift towards the chest wall.[20] Additional MRI features can include a thin septation and heterogeneous internal content within the cyst, confirming the diagnosis.[2]
Treatment / Management
Conservative Management
The treatment of a galactocele is dictated by symptom severity, lesional size, and the presence of infection. Observation is the primary management approach for most asymptomatic galactoceles, which typically spontaneously resolve. Conservative measures should be advised, including anti-inflammatory medication as needed, avoidance of over-frequent pumping, and on-demand breastfeeding or pumping. Ice packs and breast support may be helpful.[28][3][1]
Galactocele Aspiration and Catheter-Drainage
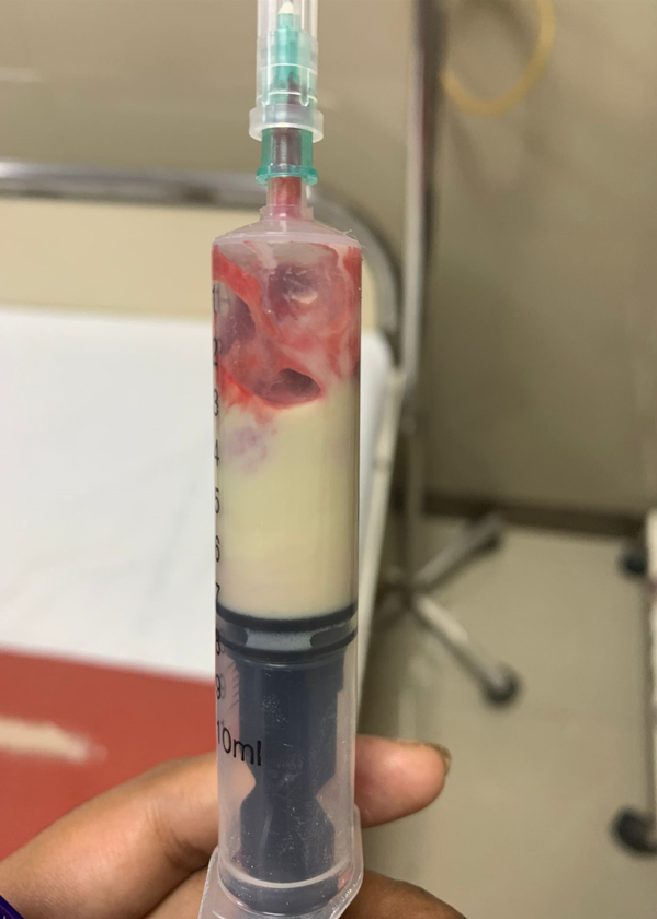
Large and symptomatic galactoceles often require intervention, typically with serial aspirations or drainage catheter placement by a breast surgeon for relief and diagnostic confirmation (see Image. Fine Needle Aspiration Specimen of Galactocele).[3] Surgical drainage is generally avoided in favor of less invasive approaches, as aspiration or catheter drainage is typically sufficient.[28] Drainage alleviates symptoms and somewhat reduces mass effect, aiding in breastfeeding.[3][1]
However, aspiration does not decrease surrounding tissue edema; cavities often refill and frequently require repeated aspiration.[28][3] Repeated aspirations can increase the risk of infection. Therefore, catheter drainage is generally preferred over multiple aspirations to prevent the conversion of a sterile galactocele to an infected one.[3]
Management of Infected Galactoceles
If infection develops, drainage and antibiotics are indicated. Initial antibiotic therapy typically includes dicloxacillin or flucloxacillin 500 mg 4 times daily for 10 to 14 days, which treats the most common infectious organisms, Staphylococcus and Streptococcus.[3][1] Cloxacillin may be administered if typical antibiotics are unavailable, although cloxacillin has variable bioavailability. Cephalexin is also an option, as it provides broader gram-negative coverage and does not require administration separate from meals.[3]
If first-line antibiotics are ineffective or contraindicated, as in cases of penicillin allergy, second-line options include clindamycin 300 mg 4 times daily for 10 to 14 days or trimethoprim-sulfamethoxazole twice daily for 10 to 14 days. However, the latter should be used cautiously in mothers of infants under 30 days old, those with G6PD deficiency, or preterm infants due to the risk of hyperbilirubinemia.[3]
Differential Diagnosis
Differential diagnoses that should be considered when evaluating patients with clinical features of a galactocele include:
- Breast cyst
- Breast abscess
- Breast carcinoma
- Fibrocystic changes
- Fibroadenomas
- Lactating adenoma
- Phlegmon
- Prominent lactiferous sinus
- Traumatic fat necrosis
- Hematoma
- Hamartoma [1]
Prognosis
Galactocele is a benign condition that typically resolves spontaneously upon cessation of lactation without any intervention. Therefore, it has an excellent prognosis. No increased risk of subsequent breast cancer or fibrocystic disease after galactocele has been reported.[29]
Complications
Inspissated Galactoceles
Galactoceles usually resolve spontaneously as the rapid hormonal changes linked to lactation stabilize. However, in some cases, desquamated epithelial cells and stagnated milk result in an inspissated cyst forming crystals. This leads to the development of a crystalizing or solid galactocele, a rare entity with only approximately 10 reported cases in the literature.[30][31][32][22] Inspissated galactocels are not easily diagnosed by ultrasound as they do not have any typical features of a galactocele and may even be mistaken for other benign or solid breast lesions (see Image. Inspissated Galactocele).
Fine-needle aspiration of inspissated galactoceles retrieves thick, chalky white material with a gritty sensation noted during aspiration. Hematoxylin and eosin staining of the aspirate reveals well-defined purple crystals. Leishman staining demonstrates discrete and polymorphic refractile crystals. These crystals are positively birefringent with surrounding amorphous proteinaceous material. Crystallized galactoceles cannot be emptied by aspiration alone and may require further intervention, like excision.[22]
Infected Galactoceles
Another complication associated with galactoceles is infection. The rich nutrient content of milk in galactocele with a possible unsterile technique of aspiration or excision may lead to acute mastitis, which can become further complicated by the formation of a breast abscess.[28] Clinically, the affected breast becomes swollen and tender, with signs of inflammation present. Staphylococcus aureus is the most common causative organism, followed by streptococci. These common pathogens are present in the nose and throat of nursing infants and infect the breast via the damaged epithelial interface of the nipple-areolar complex. An infected galactocele appears as a complex cyst with thickened walls during ultrasonography.[3]
Treatment includes intravenous antibiotics and aspiration or surgical drainage. (Please refer to "Management of Infected Galactoceles" in the Treatment section for more information on managing this complication). Sometimes, a breast implant, which is a known predisposing factor, may also get infected with an infected galactocele, necessitating implant removal along with incision and drainage of the abscess.[33][28] Breastfeeding should continue because it promotes drainage. A milk fistula is a rare complication following aspiration or drain placement of an infected galactocele or breast abscess.[3]
Consultations
Consultations for galactocele management may include:
- Radiologist
- Interventional radiologists
- General surgeon
- Lactation consultant
- Obstetrician and gynecologist
Deterrence and Patient Education
All postpartum patients and neonates must receive postnatal care within the first 24 hours. All postpartum patients should be offered help to teach the best practices in breastfeeding the newborn with the correct technique. Nurse practitioners, midwives, and lactation specialists have an essential role in the prevention, early diagnosis, and prompt referral of lactating patients with breast lumps. The nurse practitioner plays a vital role in educating the patients and their families about their condition.
Regular follow-up visits must be encouraged to ensure the health of postpartum patients and infants. Patients must be counseled about the importance of breast hygiene during lactation. Proper counseling and education of postpartum patients regarding their health cannot be overemphasized. If available, counseling and education can be performed using information leaflets and posters or referrals to educational websites.
Enhancing Healthcare Team Outcomes
An interprofessional team approach is essential in managing galactoceles, as these commonly occur in lactating patients and often resolve with conservative management. This team, composed of physicians, advanced practitioners, nurses, lactation specialists, radiologists, and pharmacists, collaborates to provide patient-centered care. Early diagnosis and treatment benefit from a holistic, integrated approach where team members leverage their expertise. Physicians, for example, can ensure a thorough assessment and involve radiologists for imaging in cases that require further investigation, particularly with unusual presentations like galactoceles in infants or nonlactating patients. When ultrasound-guided FNA is performed, communication with cytopathologists is essential for an accurate diagnosis, and effective collaboration with radiologists and pathologists is fundamental to achieving optimal outcomes.
In cases where a galactocele becomes complicated by infection, the extracted pus is cultured, and a microbiologist’s input guides antibiotic choice based on sensitivity reports, enhancing patient safety and treatment efficacy. Shared decision-making between clinicians and surgeons, with a tailored approach to drainage interventions, further supports patient-centered care. Nurses play a critical role by monitoring vital signs, educating patients and families on self-care and breastfeeding practices, and providing emotional support. This coordinated interprofessional communication and care strategy optimizes patient outcomes and team performance, ensuring patient safety remains central throughout the care continuum.