Continuing Education Activity
Corneal topography evaluates the anterior curvature of the cornea while corneal tomography includes the posterior curvature and corneal thickness. This article compares and contrasts the various methods of obtaining corneal data. Corneal imaging is most applicable when evaluating keratorefractive surgery candidacy and keratoconus-risk. This activity highlights the role of the interprofessional team in evaluating and improving care for patients with corneal pathologies.
Objectives:
- Review the principles of corneal topography and tomography, and the standard maps produced by such techniques.
- Identify the most definitive test for evaluating corneal pathologies for the intended purpose.
- Outline the risk factors for developing keratoconus or keratorefractive surgery complications.
- Review the importance of collaboration and communication among health care professionals to enhance the delivery of care for patients receiving the ophthalmic evaluation.
Introduction
Corneal imaging is widely used by ophthalmologists to understand the shape and curvature of the cornea. Corneal topography evaluates the anterior surface of the cornea and displays the information using a color-coded map. On the other hand, corneal tomography takes into account the thickness of the cornea, allowing the posterior surface of the cornea to be elucidated.[1] Their ability to track disease progression, treatment efficacy over time, and screen candidates for particular surgical procedures make them one of ophthalmology’s essential imaging studies. The goal of this article is to understand the basics of corneal topography and tomography, understand its indications, and interpret its findings.
Indications
Indications of corneal imaging are as follow:
- To determine properties of the cornea including elevation, shape, curvature, and thickness (topography)
- To determine properties of the posterior surface of the cornea and anterior chamber (optical coherence tomography)
- To evaluate and inform pre- and post-operative clinical treatment decision making
- To evaluate and monitor corneal disease progression or improvement
Contraindications
There are no contraindications to corneal topography or tomography, as these are non-invasive. Artificial tears instillation before image acquisition is cautioned, as the tear film produced can be inaccurately read as the cornea’s topography.
Equipment
Topography
Placido Disc Topography
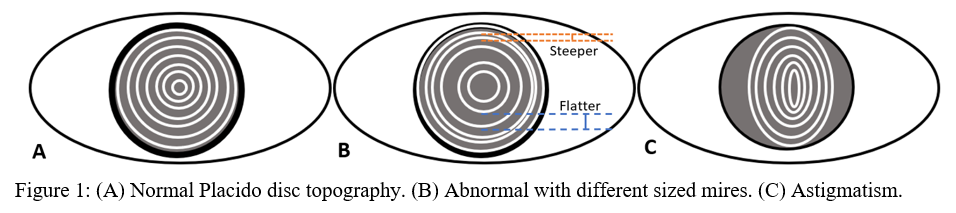
Placido disc was developed in the 1880s and was one of the first methods developed to measure the anterior corneal surface.[1] Rings of alternating dark and light circles, called mires, are directed on to the corneal surface, and its reflection is recorded (Figure 1). Depending on the shape of reflected circles, ophthalmologists could qualitatively visualize the surface of the cornea.[2] Today, sophisticated algorithms can turn the once qualitative data into more useful quantitative data. However, a significant limitation of the Placido disc is that there is no information regarding the posterior surface of the cornea. Also, it is only able to visualize 60% of the corneal surface.[1] Placido disc devices give the medical provider necessary information about the corneal surface and are used by many optometrists for routine eye exams.
Tomography
Slit-Scanning Elevation Topography
Slit-scanning elevation can image both the anterior and posterior surfaces. A slit beam of light is directed onto the cornea, and the different refractions from the anterior and posterior surface create two reflections. These two reflections, plus the original beam of light, forms a triangle, called ray-tracing triangulation, which can be interpreted using mathematical equations to recreate the surfaces of the cornea. Also, because the light reflections have the same reference point, slit-scanning elevation topography can calculate the thickness of the cornea, called corneal pachymetry.[1] Orbscan is an example of a slit-scanning device.
Scheimpflug Imaging
One limitation of slit-scanning topography is that the peripheral measurement of the cornea loses its accuracy because the object, light, and image planes are no longer parallel. This issue was addressed by taking into account the Scheimpflug principle. First used in photography, Scheimpflug noted that by extrapolating the plane on which the object and image lie, the planes would intersect at a point, producing better image resolution.[2] This is also useful for pathologies involving severe distortions of the cornea, such as in corneal ectasia, as the majority of the cornea is no longer parallel to the lens.
There are two types of devices operating with either one camera (e.g., Pentacam, Orbscan II) or with two cameras (e.g., Galilei Dual Scheimpflug Analyzer). Two cameras allow measurements to be averaged and minimize decentration, such as in the case of involuntary eye movement[1], and theoretically provide more accurate measurements, though more research is needed.[3] There are other models of Scheimpflug imaging in other countries, such as Sirius in Italy and TMS-5 in Japan.
Anterior-Segment Optical Coherence Tomography (AS-OCT)
Anterior-segment OCT produces cross-sectional images of the anterior segment, analogous to ultrasound, but instead of utilizing sound waves, it uses infrared light. A beam of light is split, one to the reference mirror at a known distance, and the second to the sample. Light scatters differently when it encounters various structures, and the OCT, called low-coherence interferometry, picks up the time delay.
Time-domain (TD) OCT has a reference mirror that moves around to scan the cornea, taking a series of light samples. Visante OCT and Heidelberg slit-lamp OCT are examples of time-domain OCTs.[4]
Fourier-domain (FD) OCT, or spectral-domain, has a fixed reference mirror and takes all the measurements simultaneously.[5] Fourier-domain OCT obtains its data faster, leading to less noise and better-focused images[2], but has a smaller field of imaging than TD OCT. Spectralis, TRVue, and Cirrus OCT are examples of Fourier-domain OCTs. FD OCT produces “ultra-high resolution” images and allows visualization of discrete layers, for example, the cornea’s epithelium.[4]
A newer type of OCT, swept-source, scans faster and can penetrate more dense cataracts and measure axial length inaccessible to other OCTs.[6] Casia SS-1000 OCT, OA-200, IOL Master 700, and DRI Triton in Japan are examples of swept-source OCTs.[4]
It is standard of care to use OCT when requiring precise corneal measurements. OCT has reproducible central corneal thickness measurements but less consistent epithelial thickness.[7]
Technique or Treatment
Interpretation
Topographic maps are color-coded and can be used to interpret shape, curvature, and elevation.
Axial or Sagittal Map:
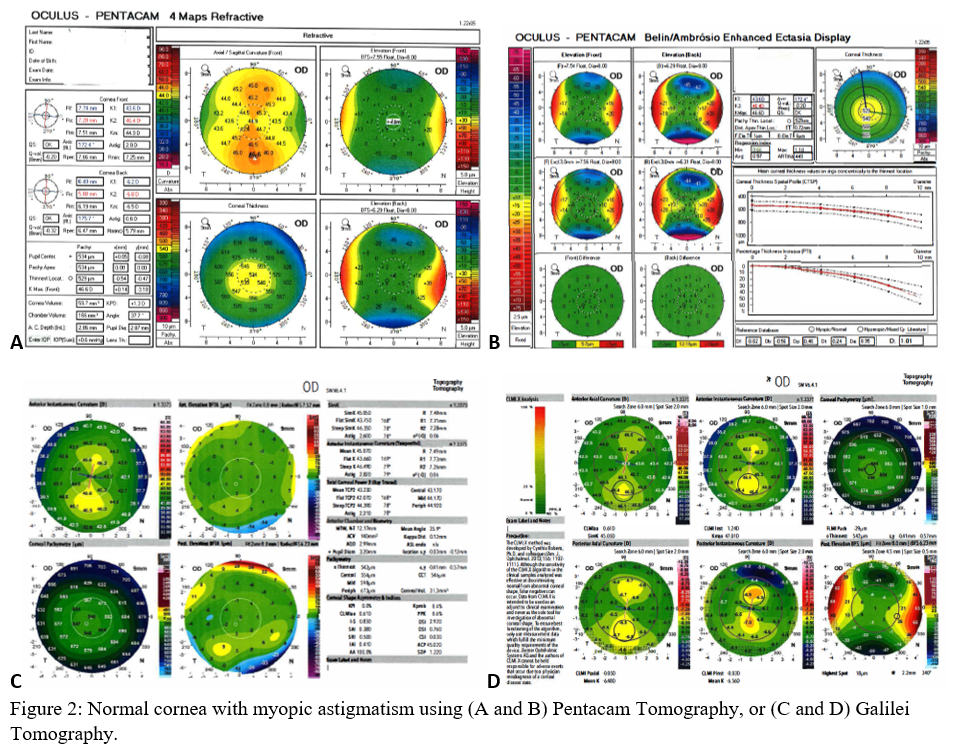
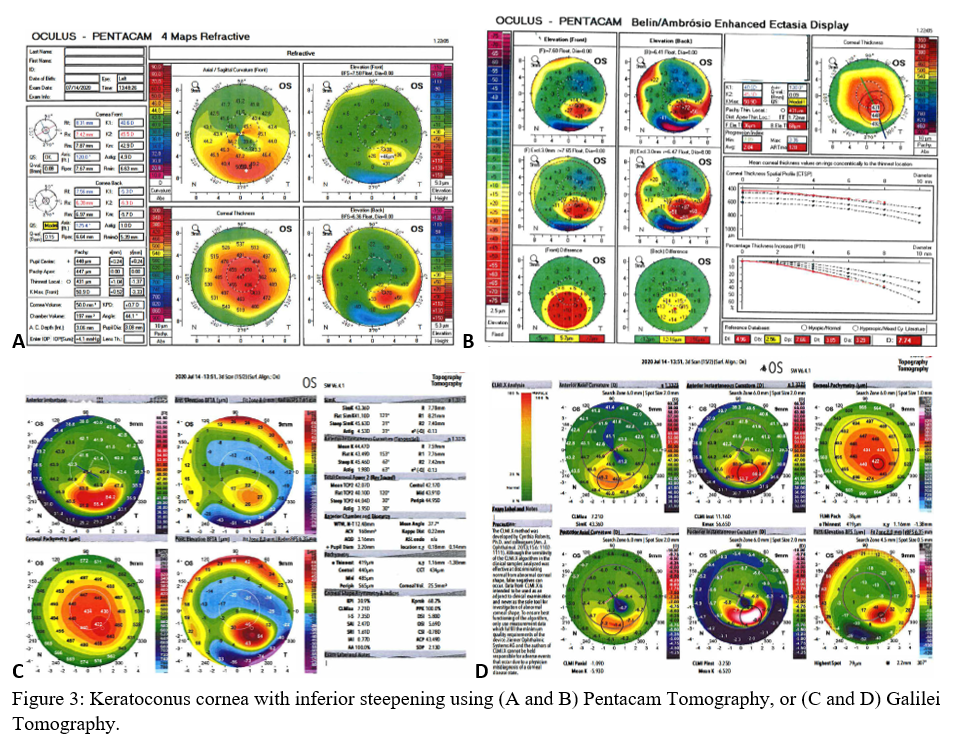
Axial maps display the distance from a particular point of the corneal surface to a reference plane in which the lens is located (i.e., if a line were to be drawn, it would cross the reference plane at a 90-degree angle).[8] It is the most often used map, capturing a two-dimensional reconstruction of the cornea. Warm colors (red, orange, yellow) represent steep areas, and cool colors (blue, green) represent flat areas. In a normal spherical cornea, most of the map will be green for its progressive, minimal flattening, with increased flattening nasally. Figure 2A (top left) is an axial map using Pentacam tomography of a normal cornea with myopic astigmatism. In a keratoconic cornea, there will be red at its peak, called the apex, with a gradual concentric color change to cool colors toward the periphery. Figure 3A (top left) is an axial map using Pentagram tomography of advanced keratoconus with inferior steepening.
Instantaneous or Tangential Map:
This map displays the slope of the corneal surface, determined by a tangential line to that point. Compared to the axial map, the instantaneous map better elucidates the changes of curvature in comparison to the rest of its surroundings and gives a better overall sense of shape.[8] These maps have the same color designation as axial maps, warm colors being steep, and cool as flat.
Elevation Map:
Elevation maps compare the corneal data to a best-fit sphere (BFS) in relation to diameter and position. An 8 to 9 mm diameter is usually chosen when evaluating for refractive surgery as a smaller or larger diameter will skew the results to be more steep or flat, respectively.[9] However, many eyes are not spherical. Therefore, the best fit toric and aspheric (BFTA) reference surface is more accurate in assessing elevation.[10] It is referred to as the best fit toric ellipsoid (BFTE). Warmer colors represent areas that are above the BFS/BFTE and cooler colors as areas below. Green is inline or similar to the BFS/BFTE.[1] Figure 2A shows the anterior (top right) and posterior (bottom right) elevation of a normal cornea with mild astigmatism using Pentacam tomography. In contrast, figure 3A is a keratoconic cornea with areas below the BFS inferiorly in both the anterior (top right) and posterior (bottom right).
Pachymetry Map:
Corneal thickness is shown on pachymetry maps. With a similar color map to the elevation map, green are the areas that are within normal limits of pachymetry. Red and warmer colors are thinner, while blue and cooler colors are thicker areas of the cornea.[1] Figure 2A (bottom left) shows mild thinning of the central cornea in a patient with myopic astigmatism. Figure 3A (bottom left) is of a keratoconus patient with more severe thinning.
Refractive Power Map:
Refractive power maps correlate the curvature of the corneal surface to the refractive function by using Snell’s Law of Refraction.[8] The refractive power map, therefore, illustrates the power, or the ability to focus light, supplied by the cornea for correcting refractive error.[11] Though not as widely used, it can demonstrate how the structure of the cornea is contributing to visual acuity.
Scheimpflug Imaging
Pentacam and Galilei are Scheimpflug imaging commonly used to identify subclinical and clinical keratoconus. There are many indices used to interpret the data and stage the degree of keratoconus. More information regarding their different specifications is discussed below under clinical significance.
AS-OCT
Rather than a coronal view, AS-OCT allows viewing of the anterior segment through the cross-section. Anatomy more posterior to the cornea can be appreciated, including the iris, pupil, and anterior chamber at different orientations. It gives a higher resolution image and can distinguish tear film, and various layers of the cornea, including the epithelial thickness.
Limitations
Evaluation using tomography and topography is non-invasive and carries no risk. However, it does require the patient to maintain a fixed gaze and can be inaccurate with eye movement.
In regards to the different methods of corneal imaging, each imaging device has its purpose and should not be used interchangeably, but are complimentary. Topography can only measure the anterior curvature of the cornea, while tomography can capture the posterior surface as well.
For example, AS-OCT is more accurate for visualizing the posterior surface of the cornea and should be used for preoperative imaging in determining surgical procedures. Slit-scanning machines, like the Orbscan, are inaccurate when measuring the posterior surface post-LASIK due to the disruption of the corneal interface, causing light scatter.[7]
Ultrasound is a gold standard when measuring central corneal thickness, and a meta-analysis found Pentacam to be clinically equivalent.[2] OCT-derived measurements are known to be higher than those taken using ultrasound (USP). It is multiplied by 0.92 to convert OCT measurement to USP; however, it is not accurate and is not recommended for preoperative use.[1] Other methods of measuring corneal thickness also had statistically significant variation.
Pentacam had lower precision when measuring axial, tangential, and refractive power maps, and pupil center corneal thickness but is still superior to Placido-disc analysis.[12]
Numerous studies compare these imaging methods from different manufacturers. These imaging instruments have their benefits and drawbacks and should be chosen based on its intended purpose.
Complications
Corneal topography and tomography are non-invasive procedures and do not have significant complications.
Clinical Significance
Keratorefractive Surgery:
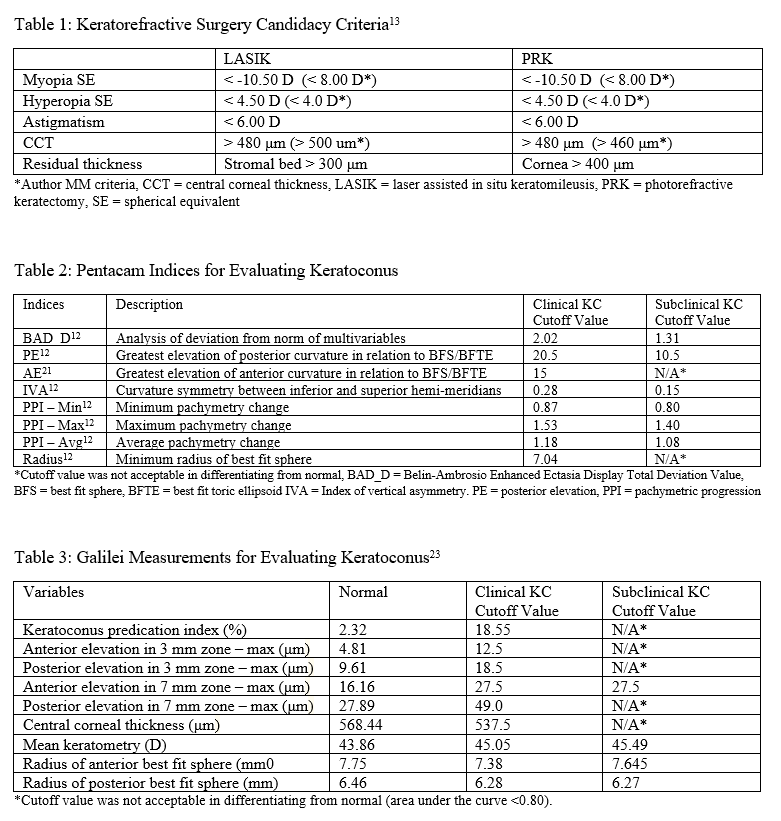
AS-OCT can be used to determine eligibility for keratorefractive surgery, such as laser-assisted in-situ keratomileusis (LASIK) or photorefractive keratectomy (PRK). Patients eligible for keratorefractive surgery must be 18 years or older, have stable refraction for a year or longer, and absent ocular and corneal pathology.[13] They must also meet corneal pachymetry criteria, seen in Table 1. Removing a portion of the cornea to correct the refractive error will result in decreased corneal rigidity and can predispose patients for corneal ectasia.[14] Keratoconus, one of the most common reasons against keratorefractive surgery recommendation, can be screened using a variety of methods and is discussed in-depth in the next section.
Central corneal thickness (CCT) < 480 μm is two standard deviations below that of a normal cornea and is contraindicated in both LASIK and PRK.[13] More recently, the percentage of corneal tissue altered has been examined to be a potential indicator of keratoconus development postoperatively. Santhiago et al. revealed that more than 40% of tissue altered significantly increased ectasia development in patients who had normal corneal topography pre-operatively, and was a more robust marker than the absolute stromal bed or preoperative CCT. This finding is expected because the anterior 40% of the central corneal stroma has significantly more tensile strength than the remaining 60%.[15] However, more research is required to examine its reliability.
Corneal topography and tomography are also used post-surgically to evaluate residual corneal structure, complications, and if reoperation would be beneficial for a patient with unsatisfactory results.
Galilei is a dual Scheimpflug system commonly used in keratorefractive surgery. By combining corneal surface data and elevation data from Placido disc topography and Scheimpflug imaging, respectively, Galilei can obtain all necessary preoperative keratorefractive surgery measurements. It also delivers excellent accuracy and has the most repeatability than other Scheimpflug devices.[3]
Keratoconus:
Keratoconus (KC) is the most common etiology of non-inflammatory thinning of the cornea, called corneal ectasia, causing distortion and conical bulging.[16] Pinero et al. modified Rabinowitz criteria and defined KC as focal areas > 47.0 D (diopters), inferior-superior index > 1.4D, or irregular curvature (asymmetric, skewed, bowtie) which skews the steepest radial axis.[12] The inferior-superior asymmetry index (I-S) is defined as the difference between the average of five points in each inferior and superior hemi-meridians. The five points in each hemi-meridian are 3.0 mm from the center and are at 30-degree intervals.
Amsler-Krumeich is an older classification system that was created before many modern, frequently used imaging techniques were developed. A new grading system, ABCD, incorporates anterior (A) and posterior (B) radii of corneal curvature, thinnest pachymetry (C), and visual acuity best-corrected for distance (D). Because the posterior elevation is included as one of its criteria for classification, it serves as a potential grading system in identifying subclinical or at-risk KC.[12] It can be used to monitor the keratoconus progression and the therapeutic efficacy of corneal cross-linking therapy.
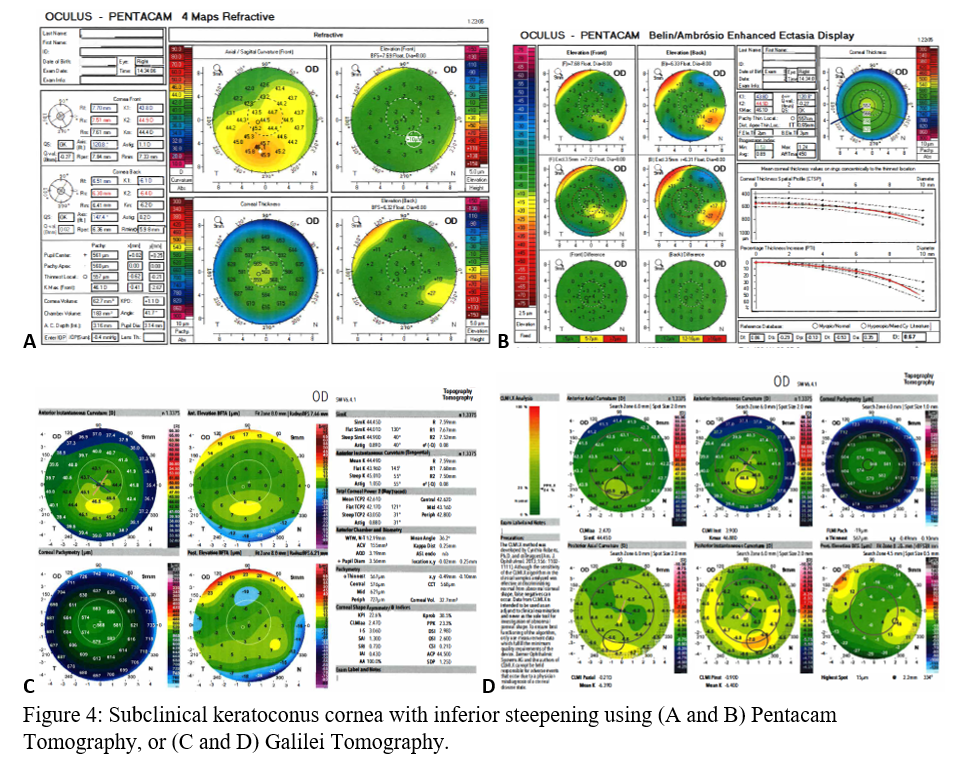
Before clinical keratoconus is diagnosed (Figures 3A-D), there are signs of subclinical keratoconus (Figures 4A-D). However, differentiating between pre-keratoconus and normal eyes is challenging. There are no widely accepted criteria for establishing such a diagnosis. Keratoconus suspect refers to patients deemed high risk for developing keratoconus based on clinical suspicion alone. Subclinical keratoconus has more tangible evidence in regards to pachymetry, curvature, and elevation though there are no standard cutoff values. Forme fruste keratoconus (FFK) has an asymmetric bowtie pattern with stable refraction and corneal curvature.[17] Using videokeratography, Li et al. define keratoconus suspect as having positive asymmetric bowtie with a skewed radial axis (AB/SRAX), and early keratoconus as the same pattern on videokeratography but also positive scissoring on retinoscopy.[18] Due to the ambiguity of such definitions, subclinical keratoconus will encompass all pre-keratoconus definitions.
When looking at the anterior surface of the cornea, the cone location and magnitude index (CLMI) uses axial and tangential maps to compare the steepest part of the cornea to the rest to evaluate for KC. The average outside the circle subtracts a 2-mm diameter circle of the steepest average of the cornea (C1), and the difference is called M1. Another 2-mm circle (C2), with the same distance from the center of the cornea as C1 (called r) 180 degrees away, is averaged and subtracted from the average outside circle C2 (labeled as M2). CLMI is then calculated as M1 – M2 if the center of C1 is located outside a 2-mm diameter circumference from the center of the cornea. If not, then CLMI is M1 – (r 3 M2). CLMI using the axial map was the strongest predictor of KC, at a threshold of 0.45.[19] CLMI.X is the CLMI index plus posterior curvature measurements. Just like the anterior measurements, posterior curvature and elevation are evaluated by taking the steepest or most elevated area and comparing it to the corresponding area, 180 degrees away. CLMI AA is the percentage of the area analyzed, or the ratio of the area used in the calculation to the area inscribed in the circle.
Corneal tomography is superior to slit-lamp examination alone in the diagnosis of both clinical and subclinical pathology.[2] It is used to monitor disease progression and also the efficacy of treatment. Non-surgical treatment involves contact lens usage, using corneal imaging to ensure proper fit and more invasive treatments, including implantation of intrastromal corneal ring segments (ICRS) or cross-linking corneal collagen. Tomography allows visualization of the ICRS and evaluation of depth-related complications such as anterior chamber perforation.[4]
When using the Pentacam system, there are many keratorefractive indices to determine keratoconus diagnosis (see Table 2, adapted from Motlagh et al.).[12] Although most are shown to be reliable, the Belin-Ambrosio Enhanced Ectasia Display Total Deviation Value (BAD_D) index seems to be best at determining subclinical or high-risk ectasia patients. (Fig. 4B). Other clinical factors are effective at detecting subclinical KC, such as pachymetric progression (PPI) and vertical asymmetry (IVA), but patient demographics should also be taken into account. Posterior elevation also performs well and should be used in conjunction with BAD_D. On the other hand, the anterior elevation is useful in detecting clinical KC but not reliable for subclinical KC and should not be used. Shown are Pentacam tomographies of a normal cornea with myopic astigmatism (Figure 2A and 2B), keratoconus cornea (Figure 3A and 3B), and a subclinical keratoconus cornea (Figure 4A and 4B).
Belin/Ambrósio Enhanced Ectasia Display (BAD) is available on Pentacam and optimizes the concept of the best fit sphere or best fit toric ellipsoid, for keratoconus detection. Called “enhanced BFS,” BAD ignores a 4 mm optical zone containing the thinnest section of the cornea and best fits the remainder of the cornea. In keratoconus, the thinnest section protrudes out and can erroneously skew results to be steeper. By excluding this zone, BAD can focus on the surrounding areas which are at risk for ectasia.[20] The BAD_D index compiles deviation from the average anterior elevation, posterior elevation, pachymetric progression, thinnest corneal thickness, displacement of thinnest region vertically, Ambrosio relational thickness to compute “D,” the total deviation value.[12]
Galilei is also used to evaluate keratoconus, and its measurements are listed in Table 3. However, cutoff values for distinguishing subclinical KC is less accurate than those for clinical KC.[21] See Galilei topographies of a normal cornea with myopic astigmatism (Figure 2C and 2D), keratoconus cornea (Figure 3C and 3D), and a subclinical keratoconus cornea (Figure 4C and 4D).
Asphericity asymmetry index (AAI) is the absolute difference between the maximum negative and maximum positive elevation value in relation to the best fit toric aspheric (BFTA) reference surface. AAI’s closer to zero means the corneal surface curvature is roughly equivalent and has little variability. AAI of the posterior corneal curvature is very reliable in detecting clinical KC, with a 100% sensitivity and 99.5% specificity. Normal posterior AAI is generally thought to be less than 20-25 μm. Though there is no standard cutoff for subclinical KC, previous studies have shown posterior AAI to be a promising index, one with a cutoff of 21.5 μm and another of 13.5 μm. However, there needs to be research to validate posterior AAI for ectasia screening further. Anterior AAI is not reliable for neither KC nor subclinical KC. AAI is also known as the Kranemann-Arce index.[3]
Cataract Surgery:
For cataract surgery, the defective cataract is removed and replaced with an artificial intraocular lens (IOL). If there is astigmatism, the patient will require a toric IOL to offset that corneal defect. Which toric lens and at which angle of implantation can be determined using corneal tomography. Postoperatively, corneal imaging can be used to detect complications such as Descemet membrane detachment and incorrect incision site closures.[4]
Glaucoma Assessment:
Using AS-OCT, information regarding the anterior chamber angle, depth, and anatomy can be obtained and potentially be used to evaluate for glaucoma risk. However, clinical evaluation and gonioscopy remain the standard.[14]
Corneal Surface Disorders:
Corneal topography is useful in tracking surface changes such as pterygiums and scars. AS-OCT, in particular, helps evaluate deeper changes to the cornea, such as foreign bodies.[14]
Corneal Transplant:
Tomography can be used to evaluate the thickness and health of the donor tissue, essential for a successful transplant. In the recipient, clear knowledge of the precise diseased layer allows less unnecessary tissue removal, resulting in a better rate of graft survival and fewer complications.[4] AS-OCT, in particular, is useful in evaluating post-operative complications such as Descemet membrane detachment, even with corneal edema, and investigating graft rejection.[14] After every keratoplasty, the corneal shape can change, causing iatrogenic astigmatism. Corneal topography and tomography are beneficial to evaluate any changes.
Enhancing Healthcare Team Outcomes
Corneal surface assessment is useful in nearly all ophthalmic evaluations. Placido disc can be used in primary care to get a general understanding of the anterior surface of the cornea and allow a large number of low-risk patients to be screened. Those with more complex pathologies can be referred to an ophthalmologist, decreasing the cost and resource usage.
Corneal pachymetry is essential in determining candidacy for keratorefractive surgery and is employed by optometrists and ophthalmologists to screen for corneal ectasia risk. Careful screening cuts down on adverse events following operation and better patient outcomes.