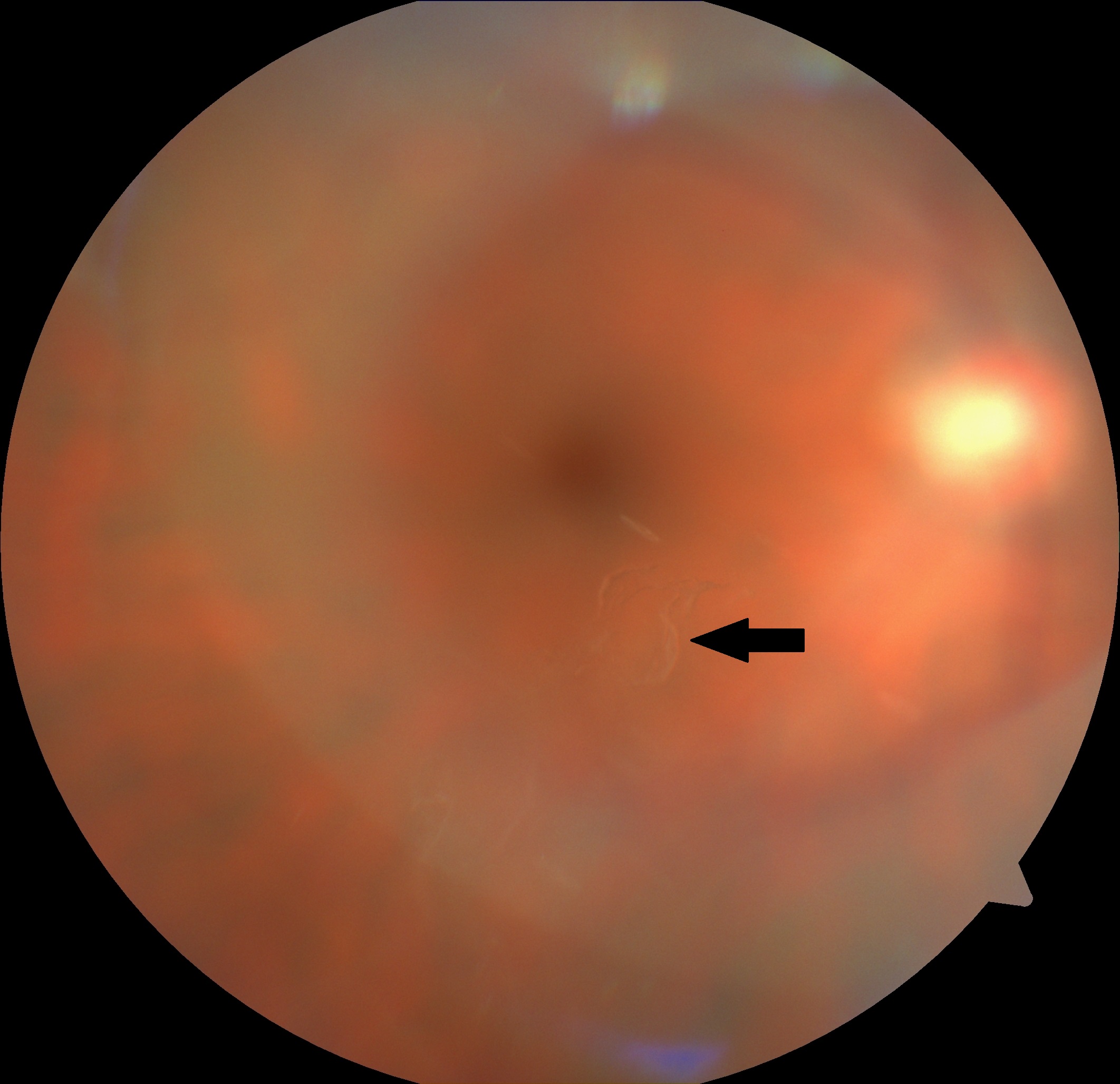
[1]
Linsenmayer TF,Gibney E,Little CD, Type II collagen in the early embryonic chick cornea and vitreous: immunoradiochemical evidence. Experimental eye research. 1982 Mar;
[PubMed PMID: 7067745]
[3]
Milston R,Madigan MC,Sebag J, Vitreous floaters: Etiology, diagnostics, and management. Survey of ophthalmology. 2016 Mar-Apr;
[PubMed PMID: 26679984]
Level 3 (low-level) evidence
[4]
Johnson MW, Posterior vitreous detachment: evolution and complications of its early stages. American journal of ophthalmology. 2010 Mar;
[PubMed PMID: 20172065]
[5]
Nuzzi R,Marchese A,Gulino GR,Versino E,Ghigo D, Influence of posterior vitreous detachment and type of intraocular lens on lipid peroxidation in the human vitreous. Molecular vision. 2015
[PubMed PMID: 26396488]
[7]
Foos RY,Wheeler NC, Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982 Dec;
[PubMed PMID: 7162795]
[8]
Bond-Taylor M,Jakobsson G,Zetterberg M, Posterior vitreous detachment - prevalence of and risk factors for retinal tears. Clinical ophthalmology (Auckland, N.Z.). 2017
[PubMed PMID: 29075095]
[9]
Coffee RE,Westfall AC,Davis GH,Mieler WF,Holz ER, Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. American journal of ophthalmology. 2007 Sep
[PubMed PMID: 17583667]
Level 2 (mid-level) evidence
[10]
Shunmugam M,Shah AN,Hysi PG,Williamson TH, The pattern and distribution of retinal breaks in eyes with rhegmatogenous retinal detachment. American journal of ophthalmology. 2014 Jan
[PubMed PMID: 24200230]
[11]
Protein-sugar interactions: preparation, purification, and properties of rabbit antibodies against di-N-acetylchitobiose., Kieda CM,Delmotte FM,Monsigny ML,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan
[PubMed PMID: 15824220]
[12]
Flaxel CJ,Adelman RA,Bailey ST,Fawzi A,Lim JI,Vemulakonda GA,Ying GS, Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration Preferred Practice Pattern®. Ophthalmology. 2020 Jan
[PubMed PMID: 31757500]
[13]
Yonemoto J,Ideta H,Sasaki K,Tanaka S,Hirose A,Oka C, The age of onset of posterior vitreous detachment. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1994 Feb;
[PubMed PMID: 8157177]
[14]
FAVRE M,GOLDMANN H, [Genesis of posterior vitreus body detachment]. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1956 Aug;
[PubMed PMID: 13378828]
[15]
Chuo JY,Lee TY,Hollands H,Morris AH,Reyes RC,Rossiter JD,Meredith SP,Maberley DA, Risk factors for posterior vitreous detachment: a case-control study. American journal of ophthalmology. 2006 Dec;
[PubMed PMID: 17157578]
Level 2 (mid-level) evidence
[16]
Hayashi K,Sato T,Manabe SI,Hirata A, Sex-Related Differences in the Progression of Posterior Vitreous Detachment with Age. Ophthalmology. Retina. 2019 Mar
[PubMed PMID: 31014700]
[17]
Morita H,Funata M,Tokoro T, A clinical study of the development of posterior vitreous detachment in high myopia. Retina (Philadelphia, Pa.). 1995;
[PubMed PMID: 7624598]
[18]
Hikichi T,Akiba J,Trempe CL, Prevalence of posterior vitreous detachment in retinitis pigmentosa. Ophthalmic surgery. 1995 Jan-Feb
[PubMed PMID: 7746622]
[19]
Ronan SM,Tran-Viet KN,Burner EL,Metlapally R,Toth CA,Young TL, Mutational hot spot potential of a novel base pair mutation of the CSPG2 gene in a family with Wagner syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 2009 Nov
[PubMed PMID: 19901218]
[20]
Hogan MJ, Inflammation and its effect on the vitreous. Transactions of the ophthalmological societies of the United Kingdom. 1975
[PubMed PMID: 1066854]
[21]
Hsu HT,Patterson R,Ryan SJ, Traumatic posterior vitreous detachment: scanning electron microscopy of an experimental model in the monkey eye. Scanning electron microscopy. 1984
[PubMed PMID: 6438790]
[22]
Coppé AM,Lapucci G, Posterior vitreous detachment and retinal detachment following cataract extraction. Current opinion in ophthalmology. 2008 May
[PubMed PMID: 18408500]
Level 3 (low-level) evidence
[23]
Sebag J,Buzney SM,Belyea DA,Kado M,McMeel JW,Trempe CL, Posterior vitreous detachment following panretinal laser photocoagulation. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1990
[PubMed PMID: 2311946]
[24]
Hesse L,Nebeling B,Schroeder B,Heller G,Kroll P, Induction of posterior vitreous detachment in rabbits by intravitreal injection of tissue plasminogen activator following cryopexy. Experimental eye research. 2000 Jan;
[PubMed PMID: 10644418]
[25]
Hendrikse F,Yeo KT, [Role of the vitreous body in diabetic retinopathy]. Klinische Monatsblatter fur Augenheilkunde. 1993 Nov
[PubMed PMID: 8114473]
[26]
Romano MR,Comune C,Ferrara M,Cennamo G,De Cillà S,Toto L,Cennamo G, Retinal Changes Induced by Epiretinal Tangential Forces. Journal of ophthalmology. 2015;
[PubMed PMID: 26421183]
[27]
Rejection by syngeneic mice of cell variants obtained by mutagenesis of a malignant teratocarcinoma cell line., Boon T,Kellermann O,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan
[PubMed PMID: 8944097]
[30]
Johnson MW, Perifoveal vitreous detachment and its macular complications. Transactions of the American Ophthalmological Society. 2005
[PubMed PMID: 17057817]
[31]
Tanner V,Harle D,Tan J,Foote B,Williamson TH,Chignell AH, Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. The British journal of ophthalmology. 2000 Nov
[PubMed PMID: 11049952]
[32]
Sebag J, Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2004 Aug;
[PubMed PMID: 15309558]
[33]
Fincham GS,James S,Spickett C,Hollingshead M,Thrasivoulou C,Poulson AV,McNinch A,Richards A,Snead D,Limb GA,Snead MP, Posterior Vitreous Detachment and the Posterior Hyaloid Membrane. Ophthalmology. 2018 Feb;
[PubMed PMID: 28867131]
[34]
Kakehashi A,Takezawa M,Akiba J, Classification of posterior vitreous detachment. Clinical ophthalmology (Auckland, N.Z.). 2014
[PubMed PMID: 24376338]
[35]
Huang D,Swanson EA,Lin CP,Schuman JS,Stinson WG,Chang W,Hee MR,Flotte T,Gregory K,Puliafito CA, Optical coherence tomography. Science (New York, N.Y.). 1991 Nov 22;
[PubMed PMID: 1957169]
[36]
Uchino E,Uemura A,Ohba N, Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Oct
[PubMed PMID: 11594947]
[37]
Chan A,Duker JS,Schuman JS,Fujimoto JG, Stage 0 macular holes: observations by optical coherence tomography. Ophthalmology. 2004 Nov
[PubMed PMID: 15522368]
[38]
van Etten PG,van Overdam KA,Reyniers R,Veckeneer M,Faridpooya K,Wubbels RJ,Manning S,La Heij EC,van Meurs JC, STRICT POSTURING WITH OR WITHOUT BILATERAL PATCHING FOR POSTERIOR VITREOUS DETACHMENT-RELATED VITREOUS HEMORRHAGE. Retina (Philadelphia, Pa.). 2020 Jun
[PubMed PMID: 31136460]
[39]
Sandinha MT,Kotagiri AK,Owen RI,Geenen C,Steel DH, Accuracy of B-scan ultrasonography in acute fundus obscuring vitreous hemorrhage using a standardized scanning protocol and a dedicated ophthalmic ultrasonographer. Clinical ophthalmology (Auckland, N.Z.). 2017
[PubMed PMID: 28794614]
[40]
Kim YK,Moon SY,Yim KM,Seong SJ,Hwang JY,Park SP, Psychological Distress in Patients with Symptomatic Vitreous Floaters. Journal of ophthalmology. 2017
[PubMed PMID: 29375909]
[41]
Henry CR,Smiddy WE,Flynn HW Jr, Pars plana vitrectomy for vitreous floaters: is there such a thing as minimally invasive vitreoretinal surgery? Retina (Philadelphia, Pa.). 2014 Jun;
[PubMed PMID: 24589875]
[42]
Tripathy K, Is Floaterectomy Worth the Risks? Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2017 May-Jun
[PubMed PMID: 28558177]
[43]
de Nie KF,Crama N,Tilanus MA,Klevering BJ,Boon CJ, Pars plana vitrectomy for disturbing primary vitreous floaters: clinical outcome and patient satisfaction. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013 May;
[PubMed PMID: 23250478]
Level 2 (mid-level) evidence
[44]
Schulz-Key S,Carlsson JO,Crafoord S, Longterm follow-up of pars plana vitrectomy for vitreous floaters: complications, outcomes and patient satisfaction. Acta ophthalmologica. 2011 Mar;
[PubMed PMID: 19860781]
[45]
Erratum: Borderud SP, Li Y, Burkhalter JE, Sheffer CE and Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. doi: 10.1002/ cncr.28811. Cancer. 2015 Mar 1;
[PubMed PMID: 25855820]
[46]
Delaney YM,Oyinloye A,Benjamin L, Nd:YAG vitreolysis and pars plana vitrectomy: surgical treatment for vitreous floaters. Eye (London, England). 2002 Jan;
[PubMed PMID: 11913884]
[47]
Sebag J, Pharmacologic vitreolysis--premise and promise of the first decade. Retina (Philadelphia, Pa.). 2009 Jul-Aug;
[PubMed PMID: 19584647]
[48]
Khoshnevis M,Sebag J, Pharmacologic vitreolysis with ocriplasmin: rationale for use and therapeutic potential in vitreo-retinal disorders. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2015 Apr
[PubMed PMID: 25812991]
[49]
Selective and accurate transcription of the Xenopus laevis 5S RNA genes in isolated chromatin by purified RNA polymerase III., Parker CS,Roeder RG,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan
[PubMed PMID: 24062204]
[50]
Takayama K,Enoki T,Kojima T,Ishikawa S,Takeuchi M, Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clinical ophthalmology (Auckland, N.Z.). 2012
[PubMed PMID: 22791965]
[51]
Emsley E,Steptoe PJ,Cazabon S, Management of a rhegmatogenous retinal detachment in a low-resource setting: treatment options when there is no vitreoretinal surgeon. BMJ case reports. 2018 Mar 28
[PubMed PMID: 29592990]
Level 3 (low-level) evidence
[52]
Chalam KV,Murthy RK,Gupta SK,Khetpal V, Prophylactic circumferential intraoperative laser retinopexy decreases the risk of retinal detachment after macular hole surgery. European journal of ophthalmology. 2012 Sep-Oct;
[PubMed PMID: 22344467]
[53]
Parolini B,Prigione G,Romanelli F,Cereda MG,Sartore M,Pertile G, Postoperative complications and intraocular pressure in 943 consecutive cases of 23-gauge transconjunctival pars plana vitrectomy with 1-year follow-up. Retina (Philadelphia, Pa.). 2010 Jan;
[PubMed PMID: 19816241]
Level 3 (low-level) evidence
[54]
Sudharshan S,Ganesh SK,Biswas J, Current approach in the diagnosis and management of posterior uveitis. Indian journal of ophthalmology. 2010 Jan-Feb
[PubMed PMID: 20029144]
[55]
Bittner AK,Diener-West M,Dagnelie G, A survey of photopsias in self-reported retinitis pigmentosa: location of photopsias is related to disease severity. Retina (Philadelphia, Pa.). 2009 Nov-Dec
[PubMed PMID: 19730162]
Level 3 (low-level) evidence