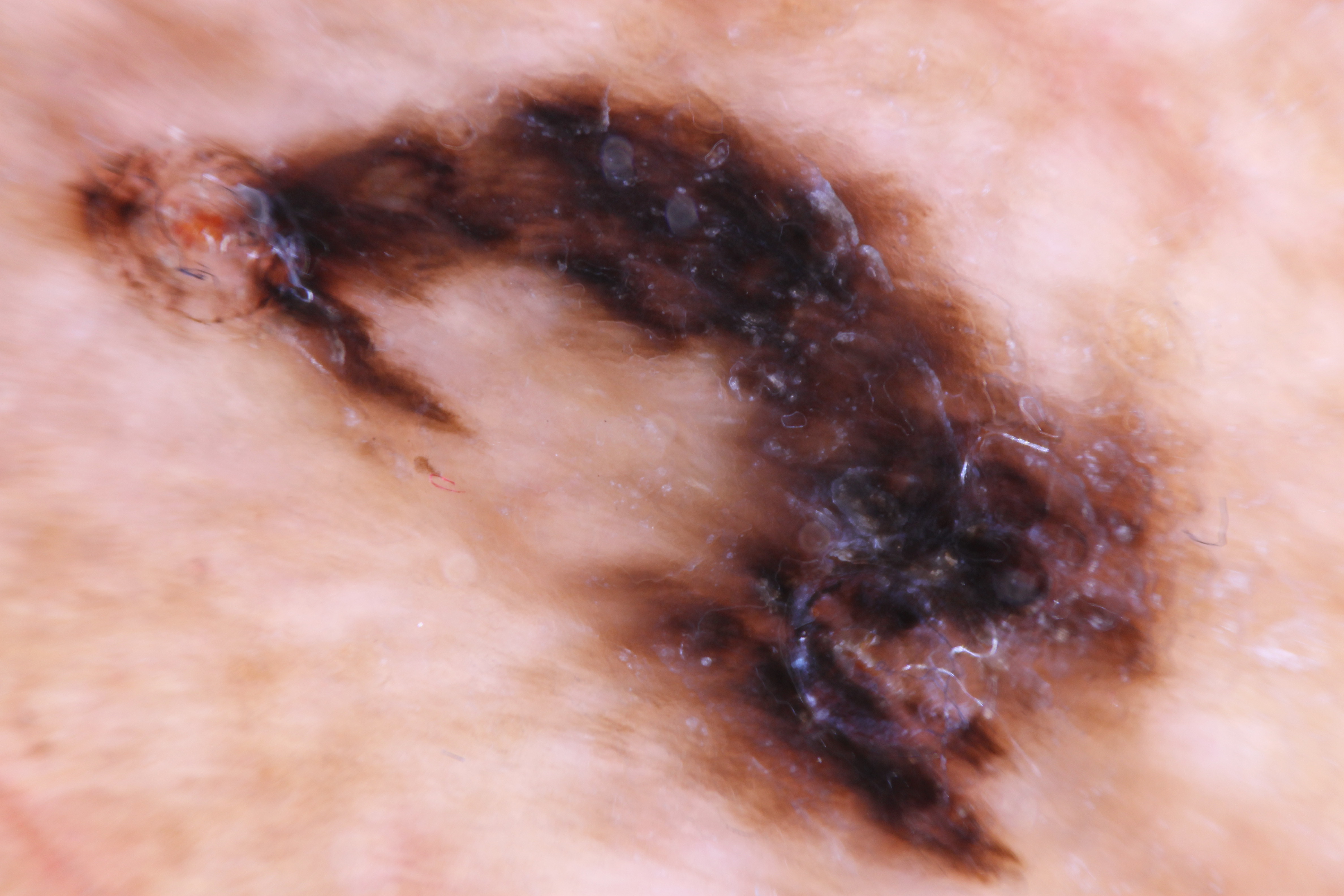
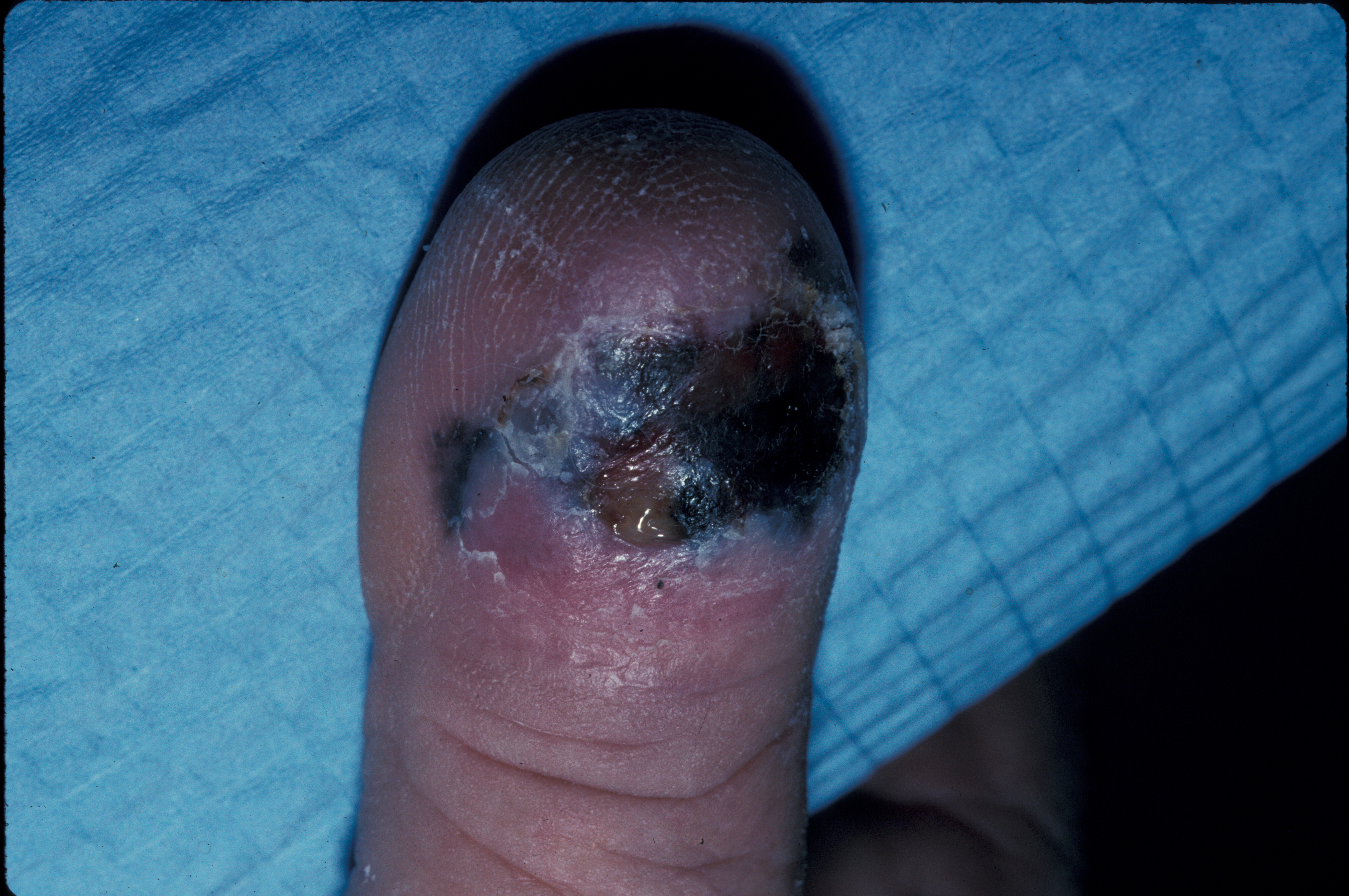
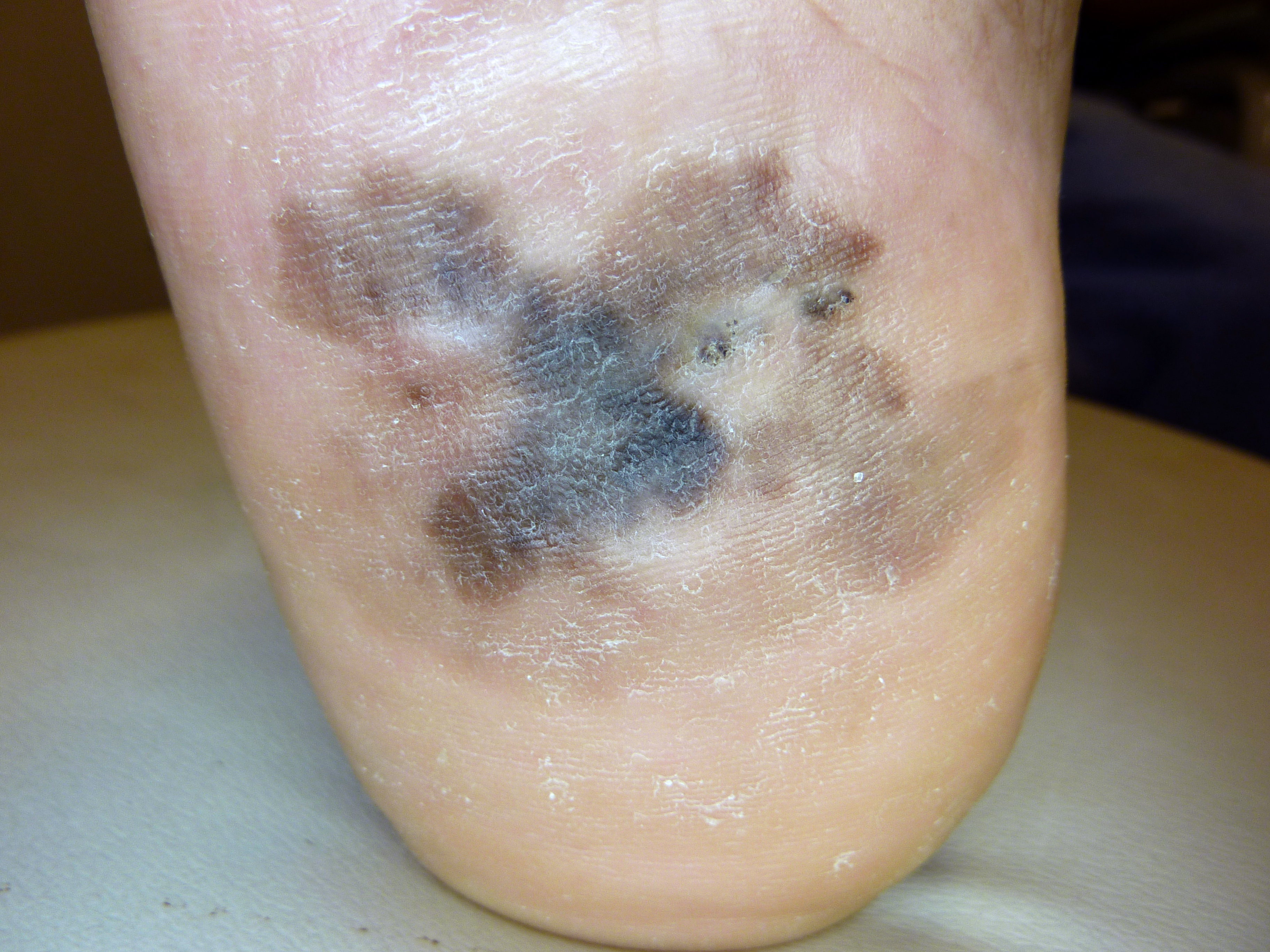
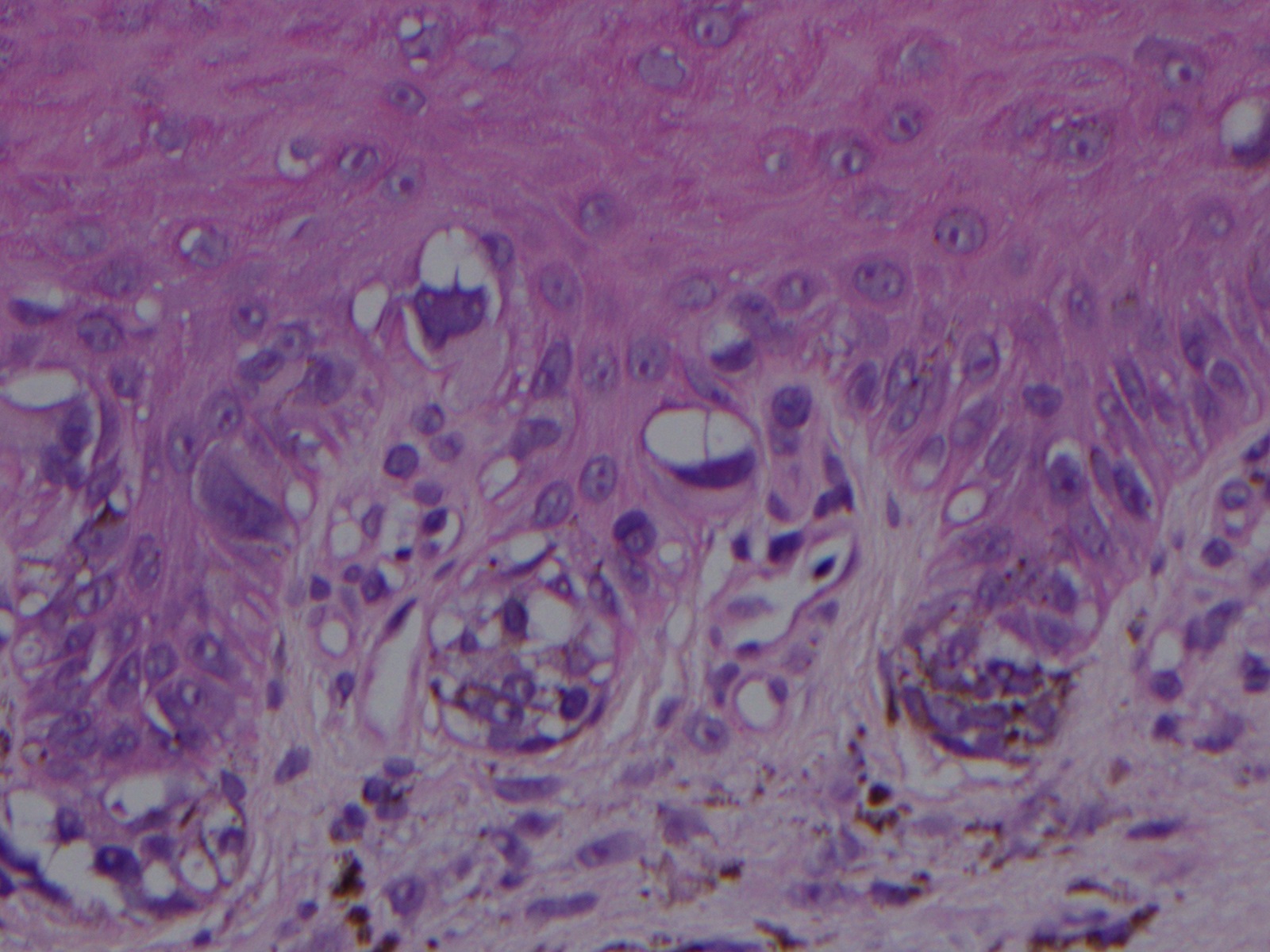
[1]
Arrington JH 3rd, Reed RJ, Ichinose H, Krementz ET. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. The American journal of surgical pathology. 1977 Jun:1(2):131-43
[PubMed PMID: 602975]
[2]
Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Archives of dermatology. 2009 Apr:145(4):427-34. doi: 10.1001/archdermatol.2008.609. Epub
[PubMed PMID: 19380664]
[3]
Huang K, Fan J, Misra S. Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006-2015, an Analysis of the SEER Registry. The Journal of surgical research. 2020 Jul:251():329-339. doi: 10.1016/j.jss.2020.02.010. Epub 2020 Mar 21
[PubMed PMID: 32208196]
[4]
Fernandez-Flores A, Cassarino DS. Histopathological diagnosis of acral lentiginous melanoma in early stages. Annals of diagnostic pathology. 2017 Feb:26():64-69. doi: 10.1016/j.anndiagpath.2016.08.005. Epub 2016 Aug 20
[PubMed PMID: 27601330]
[5]
Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA dermatology. 2013 Nov:149(11):1281-8. doi: 10.1001/jamadermatol.2013.5853. Epub
[PubMed PMID: 24067997]
[6]
Minagawa A, Omodaka T, Okuyama R. Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. The New England journal of medicine. 2016 Jun 16:374(24):2404-6. doi: 10.1056/NEJMc1512354. Epub
[PubMed PMID: 27305207]
[7]
Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, Lee CH, Chang YL, Chu CY. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Scientific reports. 2017 Jul 17:7(1):5564. doi: 10.1038/s41598-017-05809-9. Epub 2017 Jul 17
[PubMed PMID: 28717212]
[8]
Costello CM, Pittelkow MR, Mangold AR. Acral Melanoma and Mechanical Stress on the Plantar Surface of the Foot. The New England journal of medicine. 2017 Jul 27:377(4):395-396. doi: 10.1056/NEJMc1706162. Epub
[PubMed PMID: 28745985]
[9]
Ghanavatian S, Costello CM, Buras MR, Cumsky HJL, Pittelkow MR, Swanson DL, Mangold AR. Density and distribution of acral melanocytic nevi and acral melanomas on the plantar surface of the foot. Journal of the American Academy of Dermatology. 2019 Mar:80(3):790-792.e2. doi: 10.1016/j.jaad.2018.07.019. Epub 2018 Jul 25
[PubMed PMID: 30055202]
[10]
Feibleman CE, Stoll H, Maize JC. Melanomas of the palm, sole, and nailbed: a clinicopathologic study. Cancer. 1980 Dec 1:46(11):2492-504
[PubMed PMID: 7438021]
[11]
Green A, McCredie M, MacKie R, Giles G, Young P, Morton C, Jackman L, Thursfield V. A case-control study of melanomas of the soles and palms (Australia and Scotland). Cancer causes & control : CCC. 1999 Feb:10(1):21-5
[PubMed PMID: 10334638]
Level 2 (mid-level) evidence
[12]
Lundberg R, Brytting M, Dahlgren L, Kanter-Lewensohn L, Schloss L, Dalianis T, Ragnarsson-Olding B. Human herpes virus DNA is rarely detected in non-UV light-associated primary malignant melanomas of mucous membranes. Anticancer research. 2006 Sep-Oct:26(5B):3627-31
[PubMed PMID: 17094377]
[13]
Lino-Silva LS, Domínguez-Rodríguez JA, Aguilar-Romero JM, Martínez-Said H, Salcedo-Hernández RA, García-Pérez L, Herrera-Gómez Á, Cuellar-Hubbe M. Melanoma in Mexico: Clinicopathologic Features in a Population with Predominance of Acral Lentiginous Subtype. Annals of surgical oncology. 2016 Dec:23(13):4189-4194
[PubMed PMID: 27401447]
[14]
Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC, Kuo TT, Yang CH. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma research. 2004 Dec:14(6):537-41
[PubMed PMID: 15577327]
Level 3 (low-level) evidence
[15]
Wei X, Wu D, Li H, Zhang R, Chen Y, Yao H, Chi Z, Sheng X, Cui C, Bai X, Qi Z, Li K, Lan S, Chen L, Guo R, Yao X, Mao L, Lian B, Kong Y, Dai J, Tang B, Yan X, Wang X, Li S, Zhou L, Balch CM, Si L, Guo J. The Clinicopathological and Survival Profiles Comparison Across Primary Sites in Acral Melanoma. Annals of surgical oncology. 2020 Sep:27(9):3478-3485. doi: 10.1245/s10434-020-08418-5. Epub 2020 Apr 6
[PubMed PMID: 32253677]
Level 2 (mid-level) evidence
[16]
Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, Craig DW, Adkins J, Cuyugan L, Manojlovic Z, Halperin RF, Helland A, Nasser S, Legendre C, Hurley LH, Sivaprakasam K, Johnson DB, Crandall H, Busam KJ, Zismann V, Deluca V, Lee J, Sekulic A, Ariyan CE, Sosman J, Trent J. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome research. 2017 Apr:27(4):524-532. doi: 10.1101/gr.213348.116. Epub
[PubMed PMID: 28373299]
[17]
Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. Journal of cutaneous pathology. 2014 Jun:41(6):504-8. doi: 10.1111/cup.12323. Epub 2014 Apr 2
[PubMed PMID: 24588324]
[18]
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, Jakrot V, Kazakoff S, Holmes O, Leonard C, Sabarinathan R, Mularoni L, Wood S, Xu Q, Waddell N, Tembe V, Pupo GM, De Paoli-Iseppi R, Vilain RE, Shang P, Lau LMS, Dagg RA, Schramm SJ, Pritchard A, Dutton-Regester K, Newell F, Fitzgerald A, Shang CA, Grimmond SM, Pickett HA, Yang JY, Stretch JR, Behren A, Kefford RF, Hersey P, Long GV, Cebon J, Shackleton M, Spillane AJ, Saw RPM, López-Bigas N, Pearson JV, Thompson JF, Scolyer RA, Mann GJ. Whole-genome landscapes of major melanoma subtypes. Nature. 2017 May 11:545(7653):175-180. doi: 10.1038/nature22071. Epub 2017 May 3
[PubMed PMID: 28467829]
[19]
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. The New England journal of medicine. 2005 Nov 17:353(20):2135-47
[PubMed PMID: 16291983]
[20]
Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Laboratory investigation; a journal of technical methods and pathology. 2017 Jun:97(6):630-635. doi: 10.1038/labinvest.2016.147. Epub 2017 Jan 16
[PubMed PMID: 28092366]
[21]
Su J, Yu W, Liu J, Zheng J, Huang S, Wang Y, Qi S, Ma X, Chen J, Zhang Y. Fluorescence in situ hybridisation as an ancillary tool in the diagnosis of acral melanoma: a review of 44 cases. Pathology. 2017 Dec:49(7):740-749. doi: 10.1016/j.pathol.2017.08.006. Epub 2017 Oct 14
[PubMed PMID: 29037804]
Level 2 (mid-level) evidence
[22]
Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. The British journal of dermatology. 2000 Aug:143(2):275-80
[PubMed PMID: 10951133]
[23]
Boyd AS, Rapini RP. Acral melanocytic neoplasms: a histologic analysis of 158 lesions. Journal of the American Academy of Dermatology. 1994 Nov:31(5 Pt 1):740-5
[PubMed PMID: 7929919]
[24]
Scolyer RA, Thompson JF, McCarthy SW, Strutton GM, Elder DE. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. The Australasian journal of dermatology. 2006 Feb:47(1):71-3; author reply 74-5
[PubMed PMID: 16405491]
[25]
Darmawan CC, Jo G, Montenegro SE, Kwak Y, Cheol L, Cho KH, Mun JH. Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. Journal of the American Academy of Dermatology. 2019 Sep:81(3):805-812. doi: 10.1016/j.jaad.2019.01.081. Epub 2019 Feb 5
[PubMed PMID: 30731177]
[26]
Pampena R, Kyrgidis A, Lallas A, Moscarella E, Argenziano G, Longo C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. Journal of the American Academy of Dermatology. 2017 Nov:77(5):938-945.e4. doi: 10.1016/j.jaad.2017.06.149. Epub 2017 Aug 29
[PubMed PMID: 28864306]
Level 1 (high-level) evidence
[27]
Bristow IR, de Berker DA, Acland KM, Turner RJ, Bowling J. Clinical guidelines for the recognition of melanoma of the foot and nail unit. Journal of foot and ankle research. 2010 Nov 1:3():25. doi: 10.1186/1757-1146-3-25. Epub 2010 Nov 1
[PubMed PMID: 21040565]
[28]
Tan A, Stein JA. Dermoscopic patterns of acral melanocytic lesions in skin of color. Cutis. 2019 May:103(5):274-276
[PubMed PMID: 31233579]
[29]
Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Archives of dermatology. 2011 Jun:147(6):741-3. doi: 10.1001/archdermatol.2011.136. Epub
[PubMed PMID: 21690544]
[30]
Lallas A, Kyrgidis A, Koga H, Moscarella E, Tschandl P, Apalla Z, Di Stefani A, Ioannides D, Kittler H, Kobayashi K, Lazaridou E, Longo C, Phan A, Saida T, Tanaka M, Thomas L, Zalaudek I, Argenziano G. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. The British journal of dermatology. 2015 Oct:173(4):1041-9. doi: 10.1111/bjd.14045. Epub 2015 Oct 1
[PubMed PMID: 26211689]
[31]
Saida T, Yoshida N, Ikegawa S, Ishihara K, Nakajima T. Clinical guidelines for the early detection of plantar malignant melanoma. Journal of the American Academy of Dermatology. 1990 Jul:23(1):37-40
[PubMed PMID: 2365875]
[32]
Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, Sober AJ, Thompson JA, Wisco OJ, Wyatt S, Hu S, Lamina T. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology. 2019 Jan:80(1):208-250. doi: 10.1016/j.jaad.2018.08.055. Epub 2018 Nov 1
[PubMed PMID: 30392755]
[33]
Neuman HB, Patel A, Ishill N, Hanlon C, Brady MS, Halpern AC, Houghton A, Coit DG. A single-institution validation of the AJCC staging system for stage IV melanoma. Annals of surgical oncology. 2008 Jul:15(7):2034-41. doi: 10.1245/s10434-008-9915-0. Epub 2008 May 9
[PubMed PMID: 18465172]
Level 1 (high-level) evidence
[34]
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Puleo CA, Coventry BJ, Kashani-Sabet M, Smithers BM, Paul E, Kraybill WG, McKinnon JG, Wang HJ, Elashoff R, Faries MB, MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. The New England journal of medicine. 2014 Feb 13:370(7):599-609. doi: 10.1056/NEJMoa1310460. Epub
[PubMed PMID: 24521106]
[35]
Haneke E, Baran R. Longitudinal melanonychia. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Jun:27(6):580-4
[PubMed PMID: 11442597]
[36]
Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. Journal of the American Academy of Dermatology. 2003 Feb:48(2):183-8
[PubMed PMID: 12582386]
[37]
Tseng JF, Tanabe KK, Gadd MA, Cosimi AB, Malt RA, Haluska FG, Mihm MC Jr, Sober AJ, Souba WW. Surgical management of primary cutaneous melanomas of the hands and feet. Annals of surgery. 1997 May:225(5):544-50; discussion 550-3
[PubMed PMID: 9193182]
[38]
Goydos JS, Shoen SL. Acral Lentiginous Melanoma. Cancer treatment and research. 2016:167():321-9. doi: 10.1007/978-3-319-22539-5_14. Epub
[PubMed PMID: 26601870]
[39]
Roh MR, Kim J, Chung KY. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei medical journal. 2010 Jul:51(4):562-8. doi: 10.3349/ymj.2010.51.4.562. Epub
[PubMed PMID: 20499423]
[40]
Tanaka K, Nakamura Y, Mizutani T, Shibata T, Tsutsumida A, Fukuda H, Matsushita S, Aoki M, Namikawa K, Ohe S, Fukushima S, Yamazaki N, Dermatologic Oncology Group of the Japan Clinical Oncology Group. Confirmatory trial of non-amputative digit preservation surgery for subungual melanoma: Japan Clinical Oncology Group study (JCOG1602, J-NAIL study protocol). BMC cancer. 2019 Oct 25:19(1):1002. doi: 10.1186/s12885-019-6248-2. Epub 2019 Oct 25
[PubMed PMID: 31653251]
[41]
Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. The British journal of dermatology. 2011 Oct:165(4):852-8. doi: 10.1111/j.1365-2133.2011.10477.x. Epub 2011 Aug 4
[PubMed PMID: 21812768]
Level 3 (low-level) evidence
[42]
Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, Urban A, Schell H, Hohenberger W, Sauer R. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. International journal of radiation oncology, biology, physics. 1999 Jun 1:44(3):607-18
[PubMed PMID: 10348291]
[43]
Nakamura Y, Fujisawa Y. Diagnosis and Management of Acral Lentiginous Melanoma. Current treatment options in oncology. 2018 Jun 27:19(8):42. doi: 10.1007/s11864-018-0560-y. Epub 2018 Jun 27
[PubMed PMID: 29951919]
[44]
Hadash-Bengad R, Hajaj E, Klein S, Merims S, Frank S, Eisenberg G, Yakobson A, Orevi M, Caplan N, Peretz T, Lotem M, Cohen JE. Immunotherapy Potentiates the Effect of Chemotherapy in Metastatic Melanoma-A Retrospective Study. Frontiers in oncology. 2020:10():70. doi: 10.3389/fonc.2020.00070. Epub 2020 Feb 14
[PubMed PMID: 32117727]
Level 2 (mid-level) evidence
[45]
Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH, Kraft S, Gerstenblith MR, Thompson CL, Honda K, Cuda JD, Eberhart CG, Handa JT, Lipson EJ, Taube JM. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Laboratory investigation; a journal of technical methods and pathology. 2017 Sep:97(9):1063-1071. doi: 10.1038/labinvest.2017.64. Epub 2017 Jul 24
[PubMed PMID: 28737763]
[46]
Savarese I, Papi F, D'Errico A, Gori A, Grazzini M, Vannucchi M, Massi D, De Giorgi V. Acral lentiginous melanoma treated with topical imiquimod cream: possible cooperation between drug and tumour cells. Clinical and experimental dermatology. 2015 Jan:40(1):27-30. doi: 10.1111/ced.12469. Epub 2014 Sep 23
[PubMed PMID: 25252087]
[47]
Ocampo-Garza J, Gioia Di Chiacchio N, Haneke E, le Voci F, Paschoal FM. Subungual Melanoma In Situ Treated With Imiquimod 5% Cream After Conservative Surgery Recurrence. Journal of drugs in dermatology : JDD. 2017 Mar 1:16(3):268-270
[PubMed PMID: 28301623]
[48]
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O'Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Sep 10:31(26):3182-90. doi: 10.1200/JCO.2012.47.7836. Epub 2013 Jun 17
[PubMed PMID: 23775962]
[49]
Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Annals of surgical oncology. 2018 Aug:25(8):2105-2110. doi: 10.1245/s10434-018-6513-7. Epub 2018 May 30
[PubMed PMID: 29850954]
[50]
Duarte CA, Flórez JP, López HG, Meneses MX, de Vries E. Survival of acral lentiginous melanoma in the National Cancer Institute of Colombia. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 Mar:31(3):438-442. doi: 10.1111/jdv.13913. Epub 2016 Dec 4
[PubMed PMID: 27518480]
[51]
Lv J, Dai B, Kong Y, Shen X, Kong J. Acral Melanoma in Chinese: A Clinicopathological and Prognostic Study of 142 cases. Scientific reports. 2016 Aug 22:6():31432. doi: 10.1038/srep31432. Epub 2016 Aug 22
[PubMed PMID: 27545198]
Level 3 (low-level) evidence
[52]
Bello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, Ariyan CE. Prognosis of acral melanoma: a series of 281 patients. Annals of surgical oncology. 2013 Oct:20(11):3618-25. doi: 10.1245/s10434-013-3089-0. Epub 2013 Jul 10
[PubMed PMID: 23838913]
[53]
Lino-Silva LS, Zepeda-Najar C, Salcedo-Hernández RA, Martínez-Said H. Acral Lentiginous Melanoma: Survival Analysis of 715 Cases. Journal of cutaneous medicine and surgery. 2019 Jan/Feb:23(1):38-43. doi: 10.1177/1203475418800943. Epub 2018 Sep 15
[PubMed PMID: 30221995]
Level 3 (low-level) evidence