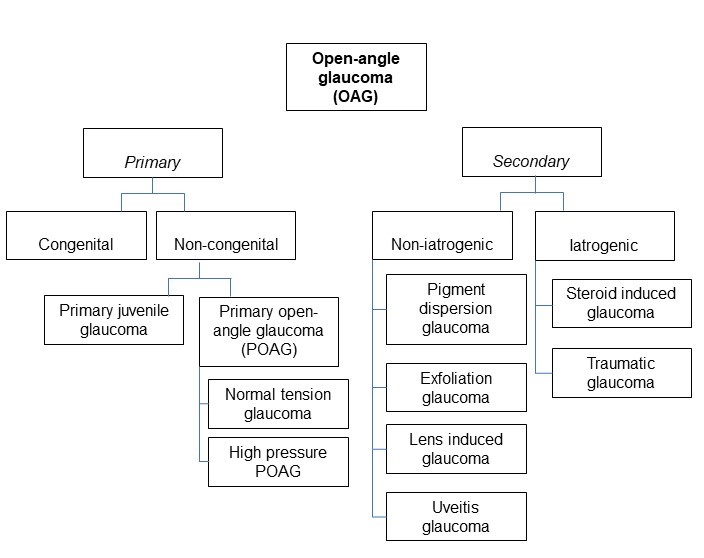
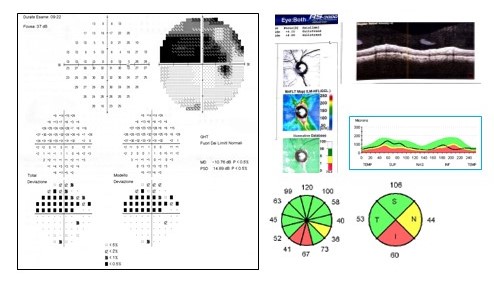
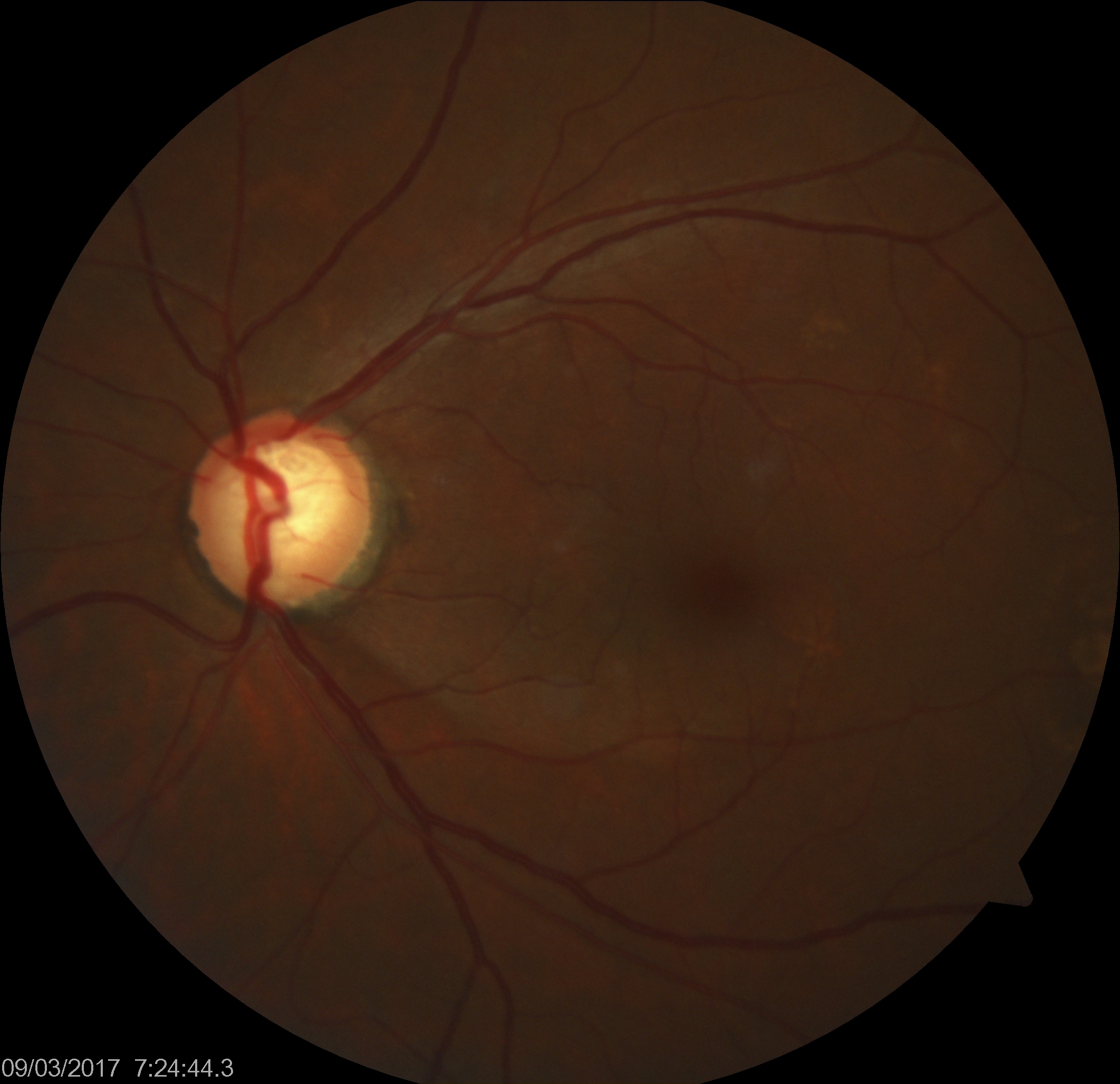
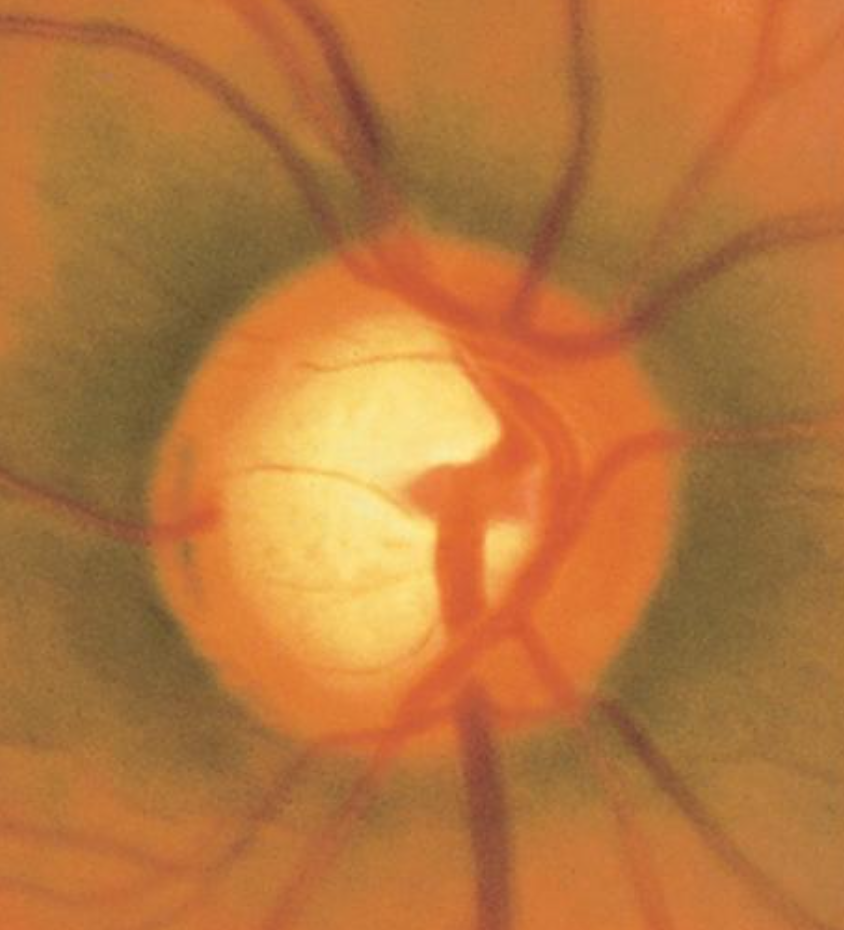
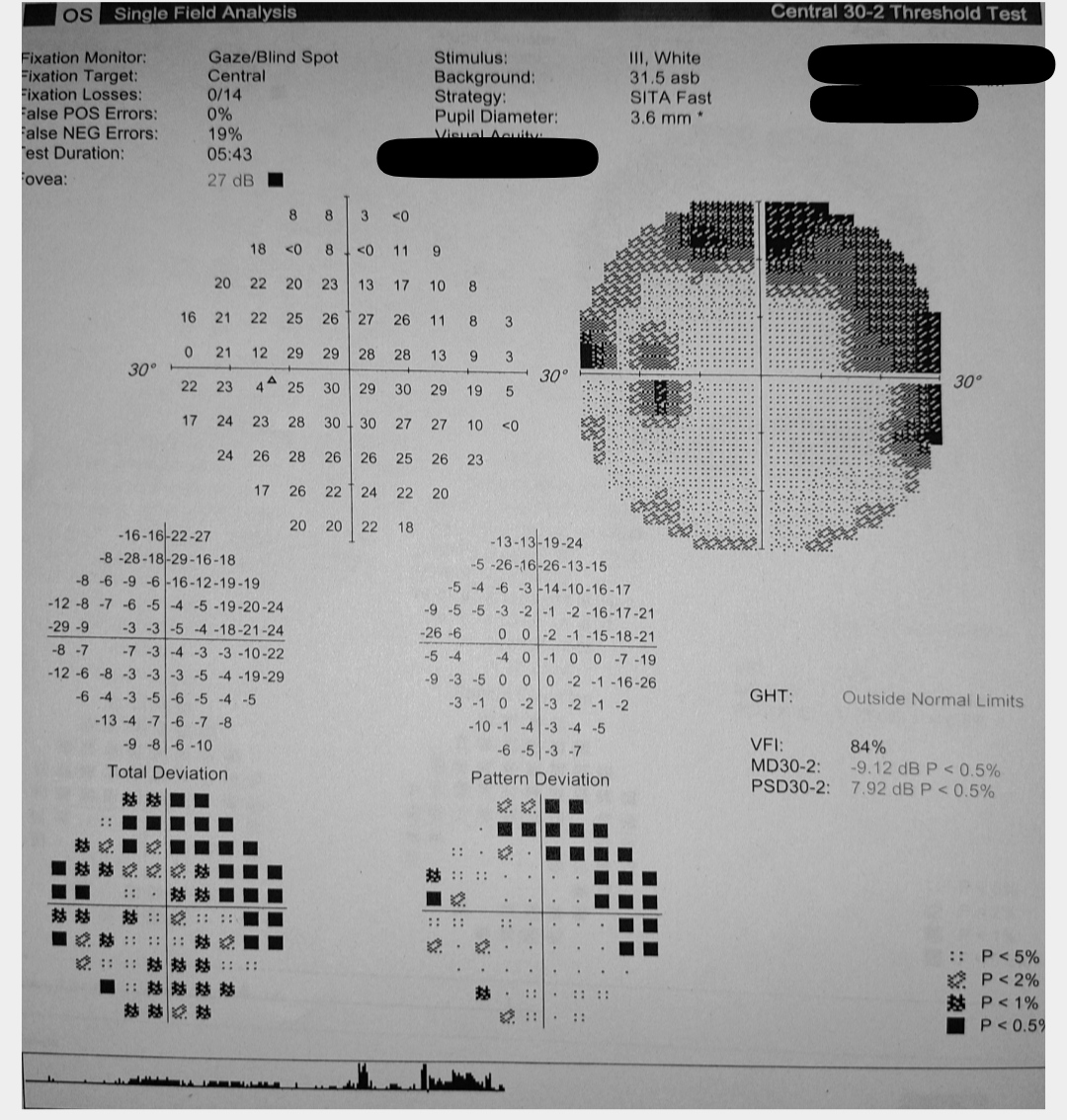
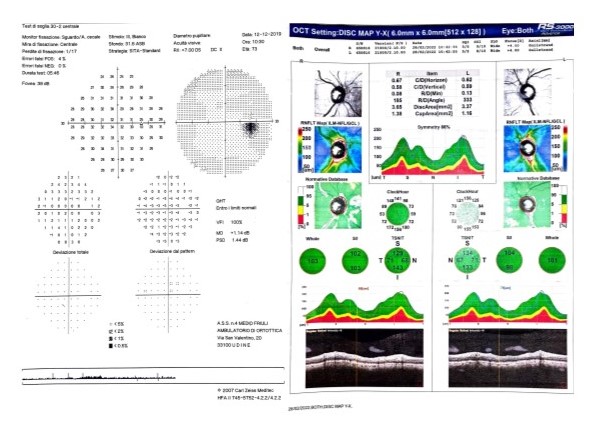
[2]
Leung DYL, Tham CC. Normal-tension glaucoma: Current concepts and approaches-A review. Clinical & experimental ophthalmology. 2022 Mar:50(2):247-259. doi: 10.1111/ceo.14043. Epub 2022 Feb 7
[PubMed PMID: 35040248]
[3]
Kastner A, King AJ. Advanced glaucoma at diagnosis: current perspectives. Eye (London, England). 2020 Jan:34(1):116-128. doi: 10.1038/s41433-019-0637-2. Epub 2019 Nov 18
[PubMed PMID: 31740802]
Level 3 (low-level) evidence
[4]
Parajuli S, Shrestha P, Sharma S, Shrestha JK. Prevalence of Ocular Hypertension in Patients Above 40 Years of Age. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2022 Jan:14(27):140-143. doi: 10.3126/nepjoph.v14i1.29740. Epub
[PubMed PMID: 35996922]
[5]
Gordon MO, Kass MA. What We Have Learned From the Ocular Hypertension Treatment Study. American journal of ophthalmology. 2018 May:189():xxiv-xxvii. doi: 10.1016/j.ajo.2018.02.016. Epub 2018 Mar 1
[PubMed PMID: 29501371]
[6]
Veeraraghavan N. Adult Eye Conditions: Primary Open-Angle Glaucoma and Cataract. FP essentials. 2022 Aug:519():19-23
[PubMed PMID: 35947132]
[7]
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14:311(18):1901-11. doi: 10.1001/jama.2014.3192. Epub
[PubMed PMID: 24825645]
[8]
Liu K, He W, Zhao J, Zeng Y, Cheng H. Association of WDR36 polymorphisms with primary open angle glaucoma: A systematic review and meta-analysis. Medicine. 2017 Jun:96(26):e7291. doi: 10.1097/MD.0000000000007291. Epub
[PubMed PMID: 28658128]
Level 1 (high-level) evidence
[9]
Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Héon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Human molecular genetics. 2005 Mar 15:14(6):725-33
[PubMed PMID: 15677485]
[10]
Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nature genetics. 2010 Oct:42(10):906-9. doi: 10.1038/ng.661. Epub 2010 Sep 12
[PubMed PMID: 20835238]
[11]
Borrás T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Progress in retinal and eye research. 2003 Jul:22(4):435-63
[PubMed PMID: 12742391]
[12]
Pasquale LR, Loomis SJ, Kang JH, Yaspan BL, Abdrabou W, Budenz DL, Chen TC, Delbono E, Friedman DS, Gaasterland D, Gaasterland T, Grosskreutz CL, Lee RK, Lichter PR, Liu Y, McCarty CA, Moroi SE, Olson LM, Realini T, Rhee DJ, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Allingham RR, Pericak-Vance MA, Weinreb RN, Zhang K, Hauser MA, Richards JE, Haines JL, Wiggs JL. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. American journal of ophthalmology. 2013 Feb:155(2):342-353.e5. doi: 10.1016/j.ajo.2012.07.023. Epub 2012 Oct 27
[PubMed PMID: 23111177]
[13]
Nunes HF, Ananina G, Costa VP, Zanchin NIT, de Vasconcellos JPC, de Melo MB. Investigation of CAV1/CAV2 rs4236601 and CDKN2B-AS1 rs2157719 in primary open-angle glaucoma patients from Brazil. Ophthalmic genetics. 2018 Apr:39(2):194-199. doi: 10.1080/13816810.2017.1393830. Epub 2017 Nov 7
[PubMed PMID: 29111846]
[14]
Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nature genetics. 2011 Jun:43(6):574-8. doi: 10.1038/ng.824. Epub 2011 May 1
[PubMed PMID: 21532571]
[15]
Shim MS, Kim KY, Noh M, Ko JY, Ahn S, An MA, Iwata T, Perkins GA, Weinreb RN, Ju WK. Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line. Biochemical and biophysical research communications. 2018 Sep 18:503(4):2690-2697. doi: 10.1016/j.bbrc.2018.08.025. Epub 2018 Aug 9
[PubMed PMID: 30100066]
[16]
Ma L, Zeng Y, Wei J, Yang D, Ding G, Liu J, Shang J, Kang Y, Ji X. Knockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018 Nov:107():1728-1735. doi: 10.1016/j.biopha.2018.08.156. Epub 2018 Sep 10
[PubMed PMID: 30257391]
[17]
Berner D, Zenkel M, Pasutto F, Hoja U, Liravi P, Gusek-Schneider GC, Kruse FE, Schödel J, Reis A, Schlötzer-Schrehardt U. Posttranscriptional Regulation of LOXL1 Expression Via Alternative Splicing and Nonsense-Mediated mRNA Decay as an Adaptive Stress Response. Investigative ophthalmology & visual science. 2017 Nov 1:58(13):5930-5940. doi: 10.1167/iovs.17-22963. Epub
[PubMed PMID: 29164236]
[18]
Guven Yilmaz S, Palamar M, Onay H, Ilim O, Aykut A, Ozkinay FF, Yagci A. Association of Lysyl Oxidase-like 1 Gene Polymorphism in Turkish Patients With Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Journal of glaucoma. 2017 Jul:26(7):684-685. doi: 10.1097/IJG.0000000000000672. Epub
[PubMed PMID: 28369001]
[19]
Sharma S, Martin S, Sykes MJ, Dave A, Hewitt AW, Burdon KP, Ronci M, Voelcker NH, Craig JE. Biological effect of LOXL1 coding variants associated with pseudoexfoliation syndrome. Experimental eye research. 2016 May:146():212-223. doi: 10.1016/j.exer.2016.03.013. Epub 2016 Mar 18
[PubMed PMID: 26997634]
[20]
Gao XR, Chiariglione M, Arch AJ. Whole-exome sequencing study identifies rare variants and genes associated with intraocular pressure and glaucoma. Nature communications. 2022 Nov 30:13(1):7376. doi: 10.1038/s41467-022-35188-3. Epub 2022 Nov 30
[PubMed PMID: 36450729]
[21]
Mabuchi F, Mabuchi N, Sakurada Y, Yoneyama S, Kashiwagi K, Yamagata Z, Takamoto M, Aihara M, Iwata T, Hashimoto K, Sato K, Shiga Y, Nakazawa T, Akiyama M, Kawase K, Ozaki M, Araie M. Genetic variants associated with glaucomatous visual field loss in primary open-angle glaucoma. Scientific reports. 2022 Dec 1:12(1):20744. doi: 10.1038/s41598-022-24915-x. Epub 2022 Dec 1
[PubMed PMID: 36456827]
[22]
Zukerman R, Harris A, Oddone F, Siesky B, Verticchio Vercellin A, Ciulla TA. Glaucoma Heritability: Molecular Mechanisms of Disease. Genes. 2021 Jul 27:12(8):. doi: 10.3390/genes12081135. Epub 2021 Jul 27
[PubMed PMID: 34440309]
[23]
Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Scientific reports. 2021 Jul 2:11(1):13762. doi: 10.1038/s41598-021-92971-w. Epub 2021 Jul 2
[PubMed PMID: 34215769]
Level 1 (high-level) evidence
[24]
Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology. 2014 Jan:121(1):134-141. doi: 10.1016/j.ophtha.2013.09.003. Epub 2013 Oct 25
[PubMed PMID: 24823760]
[25]
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology. 2006 Mar:90(3):262-7
[PubMed PMID: 16488940]
[27]
Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Archives of ophthalmology (Chicago, Ill. : 1960). 1998 Dec:116(12):1640-5
[PubMed PMID: 9869795]
[28]
Birhanu G, Tegegne AS. Predictors for elevation of Intraocular Pressure (IOP) on glaucoma patients; a retrospective cohort study design. BMC ophthalmology. 2022 Jun 7:22(1):254. doi: 10.1186/s12886-022-02431-w. Epub 2022 Jun 7
[PubMed PMID: 35672680]
Level 2 (mid-level) evidence
[29]
Ha A, Kim CY, Shim SR, Chang IB, Kim YK. Degree of Myopia and Glaucoma Risk: A Dose-Response Meta-analysis. American journal of ophthalmology. 2022 Apr:236():107-119. doi: 10.1016/j.ajo.2021.10.007. Epub 2021 Oct 11
[PubMed PMID: 34648776]
Level 1 (high-level) evidence
[30]
Ikeda Y, Mori K, Maruyama Y, Ueno M, Yoshii K, Yamamoto Y, Imai K, Omi N, Sato R, Sato F, Nakano M, Hamuro J, Tashiro K, Sotozono C, Kinoshita S. Novel Vertical Cup-to-Disc Classification to Identify Normal Eyes That Maintain Non-Glaucoma Status: A 10-Year Longitudinal Study. Journal of glaucoma. 2023 Feb 1:32(2):127-132. doi: 10.1097/IJG.0000000000002109. Epub 2022 Aug 25
[PubMed PMID: 36001508]
[31]
Lee EJ, Kee HJ, Han JC, Kee C. Evidence-based understanding of disc hemorrhage in glaucoma. Survey of ophthalmology. 2021 May-Jun:66(3):412-422. doi: 10.1016/j.survophthal.2020.09.001. Epub 2020 Sep 17
[PubMed PMID: 32949554]
Level 3 (low-level) evidence
[32]
Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. European journal of ophthalmology. 2005 Mar-Apr:15(2):196-201
[PubMed PMID: 15812759]
[33]
Kim KE, Oh S, Baek SU, Ahn SJ, Park KH, Jeoung JW. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Scientific reports. 2020 Jun 22:10(1):10056. doi: 10.1038/s41598-020-66914-w. Epub 2020 Jun 22
[PubMed PMID: 32572072]
Level 1 (high-level) evidence
[34]
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008 Jan:115(1):85-93
[PubMed PMID: 17629563]
[35]
Prum BE Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, Herndon LW Jr, Lim MC, Williams RD. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016 Jan:123(1):P41-P111. doi: 10.1016/j.ophtha.2015.10.053. Epub 2015 Nov 12
[PubMed PMID: 26581556]
[36]
Li Y, Mitchell W, Elze T, Zebardast N. Association Between Diabetes, Diabetic Retinopathy, and Glaucoma. Current diabetes reports. 2021 Sep 8:21(10):38. doi: 10.1007/s11892-021-01404-5. Epub 2021 Sep 8
[PubMed PMID: 34495413]
[37]
Matsuda A, Hara T, Miyata K, Matsuo H, Murata H, Mayama C, Asaoka R. Do pattern deviation values accurately estimate glaucomatous visual field damage in eyes with glaucoma and cataract? The British journal of ophthalmology. 2015 Sep:99(9):1240-4. doi: 10.1136/bjophthalmol-2014-306019. Epub 2015 Mar 20
[PubMed PMID: 25795915]
[38]
Gramer G, Weber BH, Gramer E. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients With Glaucoma or Ocular Hypertension. Investigative ophthalmology & visual science. 2015 Dec:56(13):7999-8007. doi: 10.1167/iovs.15-17274. Epub
[PubMed PMID: 26720447]
[39]
Baneke AJ, Aubry J, Viswanathan AC, Plant GT. The role of intracranial pressure in glaucoma and therapeutic implications. Eye (London, England). 2020 Jan:34(1):178-191. doi: 10.1038/s41433-019-0681-y. Epub 2019 Nov 27
[PubMed PMID: 31776450]
[40]
Hogden K, Mikelberg F, Sodhi M, Khosrow-Khavar F, Mansournia MA, Kezouh A, Etminan M. The association between hormonal contraceptive use and glaucoma in women of reproductive age. British journal of clinical pharmacology. 2021 Dec:87(12):4780-4785. doi: 10.1111/bcp.14920. Epub 2021 Jun 22
[PubMed PMID: 34159623]
[41]
Moreno-Montañés J, Gándara E, Gutierrez-Ruiz I, Moreno-Galarraga L, Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MÁ, Fernandez-Montero A. Healthy Lifestyle Score and Incidence of Glaucoma: The Sun Project. Nutrients. 2022 Feb 12:14(4):. doi: 10.3390/nu14040779. Epub 2022 Feb 12
[PubMed PMID: 35215429]
[42]
Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investigative ophthalmology & visual science. 2000 Oct:41(11):3460-6
[PubMed PMID: 11006239]
[43]
Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Archiv : European journal of physiology. 2007 Aug:454(5):849-55
[PubMed PMID: 17351786]
[44]
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. The Journal of comparative neurology. 2006 Jul 20:497(3):326-49
[PubMed PMID: 16736474]
Level 2 (mid-level) evidence
[45]
Morgan-Davies J, Taylor N, Hill AR, Aspinall P, O'Brien CJ, Azuara-Blanco A. Three dimensional analysis of the lamina cribrosa in glaucoma. The British journal of ophthalmology. 2004 Oct:88(10):1299-304
[PubMed PMID: 15377555]
[46]
Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Archives of ophthalmology (Chicago, Ill. : 1960). 1981 Jan:99(1):137-43
[PubMed PMID: 7458737]
[47]
Morgan-Davies J, King AJ, Aspinall P, O'Brien CJ. Measurement of a novel optic disc topographic parameter, "spikiness", in glaucoma. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2000 Aug:238(8):669-76
[PubMed PMID: 11011687]
[48]
Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994 Nov:101(11):1851-5
[PubMed PMID: 7800368]
[49]
Zhang SH, Zhao JL, Wu C. [Microcirculation of optic nerve head and glaucoma]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2016 Jun 11:52(6):466-70. doi: 10.3760/cma.j.issn.0412-4081.2016.06.018. Epub
[PubMed PMID: 27373574]
[50]
Fortune B, Reynaud J, Hardin C, Wang L, Sigal IA, Burgoyne CF. Experimental Glaucoma Causes Optic Nerve Head Neural Rim Tissue Compression: A Potentially Important Mechanism of Axon Injury. Investigative ophthalmology & visual science. 2016 Aug 1:57(10):4403-11. doi: 10.1167/iovs.16-20000. Epub
[PubMed PMID: 27564522]
[51]
Buffault J, Labbé A, Hamard P, Brignole-Baudouin F, Baudouin C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal francais d'ophtalmologie. 2020 Sep:43(7):e217-e230. doi: 10.1016/j.jfo.2020.05.002. Epub 2020 Jun 16
[PubMed PMID: 32561029]
[52]
Zukerman R, Harris A, Vercellin AV, Siesky B, Pasquale LR, Ciulla TA. Molecular Genetics of Glaucoma: Subtype and Ethnicity Considerations. Genes. 2020 Dec 31:12(1):. doi: 10.3390/genes12010055. Epub 2020 Dec 31
[PubMed PMID: 33396423]
[53]
Nishida T, Moghimi S, Chang AC, Walker E, Liebmann JM, Fazio MA, Girkin CA, Zangwill LM, Weinreb RN. Association of Intraocular Pressure With Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA ophthalmology. 2022 Dec 1:140(12):1209-1216. doi: 10.1001/jamaophthalmol.2022.4462. Epub
[PubMed PMID: 36301523]
[54]
Rao HL, Dasari S, Puttaiah NK, Pradhan ZS, Moghimi S, Mansouri K, Webers CAB, Weinreb RN. Optical Microangiography and Progressive Retinal Nerve Fiber Layer Loss in Primary Open Angle Glaucoma. American journal of ophthalmology. 2022 Jan:233():171-179. doi: 10.1016/j.ajo.2021.07.023. Epub 2021 Jul 25
[PubMed PMID: 34320375]
[55]
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Archives of ophthalmology (Chicago, Ill. : 1960). 1981 Apr:99(4):635-49
[PubMed PMID: 6164357]
[56]
Călugăru D, Mang A, Călugăru M. Secondary open-angle pigmentary glaucoma resulting from pseudophakia. Case report. Romanian journal of ophthalmology. 2016 Apr-Jun:60(2):125-130
[PubMed PMID: 29450335]
Level 3 (low-level) evidence
[57]
Gil P, Pires J, Matos R, Cardoso MS, Lopes N, Matias J, Mariano M. Corneal Elevation Topography in Primary Open Angle Glaucoma. Journal of glaucoma. 2017 Feb:26(2):e41-e45. doi: 10.1097/IJG.0000000000000535. Epub
[PubMed PMID: 27599178]
[58]
Alamri A, Alkatan H, Aljadaan I. Traumatic Ghost Cell Glaucoma with Successful Resolution of Corneal Blood Staining Following Pars Plana Vitrectomy. Middle East African journal of ophthalmology. 2016 Jul-Sep:23(3):271-3. doi: 10.4103/0974-9233.180778. Epub
[PubMed PMID: 27555716]
[60]
Zeppieri M. Pigment dispersion syndrome: A brief overview. Journal of clinical and translational research. 2022 Oct 31:8(5):344-350
[PubMed PMID: 36518550]
Level 3 (low-level) evidence
[61]
Padhy B, Alone DP. Is pseudoexfoliation glaucoma a neurodegenerative disorder? Journal of biosciences. 2021:46():. pii: 97. Epub
[PubMed PMID: 34785624]
[62]
McMenamin PG, Lee WR. Ultrastructural pathology of melanomalytic glaucoma. The British journal of ophthalmology. 1986 Dec:70(12):895-906
[PubMed PMID: 3801367]
[64]
Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA. 2021 Jan 12:325(2):164-174. doi: 10.1001/jama.2020.21899. Epub
[PubMed PMID: 33433580]
[65]
Brusini P, Zeppieri M, Tosoni C, Parisi L, Salvetat ML. Optic disc damage staging system. Journal of glaucoma. 2010 Sep:19(7):442-9. doi: 10.1097/IJG.0b013e3181ca7303. Epub
[PubMed PMID: 20051883]
[66]
Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Seminars in ophthalmology. 2017:32(2):237-250. doi: 10.3109/08820538.2015.1045150. Epub 2015 Jul 6
[PubMed PMID: 26148300]
[67]
Maupin E, Baudin F, Arnould L, Seydou A, Binquet C, Bron AM, Creuzot-Garcher CP. Accuracy of the ISNT rule and its variants for differentiating glaucomatous from normal eyes in a population-based study. The British journal of ophthalmology. 2020 Oct:104(10):1412-1417. doi: 10.1136/bjophthalmol-2019-315554. Epub 2020 Jan 20
[PubMed PMID: 31959590]
[68]
Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 2013 May 15:309(19):2035-42. doi: 10.1001/jama.2013.5099. Epub
[PubMed PMID: 23677315]
Level 1 (high-level) evidence
[69]
Susanna R Jr, Vessani RM. Staging glaucoma patient: why and how? The open ophthalmology journal. 2009 Sep 17:3():59-64. doi: 10.2174/1874364100903020059. Epub 2009 Sep 17
[PubMed PMID: 19834563]
[70]
Kalyani VK, Bharucha KM, Goyal N, Deshpande MM. Comparison of diagnostic ability of standard automated perimetry, short wavelength automated perimetry, retinal nerve fiber layer thickness analysis and ganglion cell layer thickness analysis in early detection of glaucoma. Indian journal of ophthalmology. 2021 May:69(5):1108-1112. doi: 10.4103/ijo.IJO_2409_20. Epub
[PubMed PMID: 33913843]
[71]
Zeppieri M, Brusini P, Parisi L, Johnson CA, Sampaolesi R, Salvetat ML. Pulsar perimetry in the diagnosis of early glaucoma. American journal of ophthalmology. 2010 Jan:149(1):102-12. doi: 10.1016/j.ajo.2009.07.020. Epub 2009 Oct 2
[PubMed PMID: 19800607]
[72]
Salvetat ML, Zeppieri M, Parisi L, Johnson CA, Sampaolesi R, Brusini P. Learning effect and test-retest variability of pulsar perimetry. Journal of glaucoma. 2013 Mar:22(3):230-7. doi: 10.1097/IJG.0b013e318237bfe7. Epub
[PubMed PMID: 22027935]
[73]
Salvetat ML, Zeppieri M, Parisi L, Brusini P. Rarebit perimetry in normal subjects: test-retest variability, learning effect, normative range, influence of optical defocus, and cataract extraction. Investigative ophthalmology & visual science. 2007 Nov:48(11):5320-31
[PubMed PMID: 17962489]
[74]
Brusini P, Salvetat ML, Parisi L, Zeppieri M. Probing glaucoma visual damage by rarebit perimetry. The British journal of ophthalmology. 2005 Feb:89(2):180-4
[PubMed PMID: 15665349]
[75]
Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L, Felletti M. Visual field testing with the new Humphrey Matrix: a comparison between the FDT N-30 and Matrix N-30-F tests. Acta ophthalmologica Scandinavica. 2006 Jun:84(3):351-6
[PubMed PMID: 16704697]
[76]
Mastropasqua L, Brusini P, Carpineto P, Ciancaglini M, Di Antonio L, Zeppieri MW, Parisi L. Humphrey matrix frequency doubling technology perimetry and optical coherence tomography measurement of the retinal nerve fiber layer thickness in both normal and ocular hypertensive subjects. Journal of glaucoma. 2006 Aug:15(4):328-35
[PubMed PMID: 16865011]
[77]
Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Jun:124(6):853-9
[PubMed PMID: 16769839]
[78]
Harwerth RS, Carter-Dawson L, Shen F, Smith EL 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Investigative ophthalmology & visual science. 1999 Sep:40(10):2242-50
[PubMed PMID: 10476789]
[79]
Shiga Y, Aizawa N, Tsuda S, Yokoyama Y, Omodaka K, Kunikata H, Yasui T, Kato K, Kurashima H, Miyamoto E, Hashimoto M, Nakazawa T. Preperimetric Glaucoma Prospective Study (PPGPS): Predicting Visual Field Progression With Basal Optic Nerve Head Blood Flow in Normotensive PPG Eyes. Translational vision science & technology. 2018 Jan:7(1):11. doi: 10.1167/tvst.7.1.11. Epub 2018 Jan 23
[PubMed PMID: 29372113]
[81]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27
[PubMed PMID: 34501306]
[83]
Campbell P, Edgar DF, Shah R. Inter-optometrist variability of IOP measurement for modern tonometers and their agreement with Goldmann Applanation Tonometry. Clinical & experimental optometry. 2021 Jul:104(5):602-610. doi: 10.1080/08164622.2021.1878831. Epub 2021 Feb 28
[PubMed PMID: 33689641]
[84]
Biswas S, Biswas P. Relationship between Diurnal Variation in Intraocular Pressure and Central Corneal Power. Optometry and vision science : official publication of the American Academy of Optometry. 2023 Jan 1:100(1):96-104. doi: 10.1097/OPX.0000000000001974. Epub 2022 Dec 13
[PubMed PMID: 36705719]
[85]
Belovay GW, Goldberg I. The thick and thin of the central corneal thickness in glaucoma. Eye (London, England). 2018 May:32(5):915-923. doi: 10.1038/s41433-018-0033-3. Epub 2018 Feb 15
[PubMed PMID: 29445115]
[86]
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. The British journal of ophthalmology. 2002 Feb:86(2):238-42
[PubMed PMID: 11815354]
Level 3 (low-level) evidence
[88]
Bhat KS, Reddy MV, Pai V. Correlation of retinal nerve fiber layer thickness with perimetric staging in primary open-angle glaucoma - A cross-sectional study. Oman journal of ophthalmology. 2022 Jan-Apr:15(1):36-42. doi: 10.4103/ojo.ojo_345_20. Epub 2022 Mar 2
[PubMed PMID: 35388245]
Level 2 (mid-level) evidence
[89]
Kansal V, Armstrong JJ, Pintwala R, Hutnik C. Optical coherence tomography for glaucoma diagnosis: An evidence based meta-analysis. PloS one. 2018:13(1):e0190621. doi: 10.1371/journal.pone.0190621. Epub 2018 Jan 4
[PubMed PMID: 29300765]
Level 1 (high-level) evidence
[90]
Sihota R, Angmo D, Ramaswamy D, Dada T. Simplifying "target" intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian journal of ophthalmology. 2018 Apr:66(4):495-505. doi: 10.4103/ijo.IJO_1130_17. Epub
[PubMed PMID: 29582808]
[91]
Sit AJ, Pruet CM. Personalizing Intraocular Pressure: Target Intraocular Pressure in the Setting of 24-Hour Intraocular Pressure Monitoring. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2016 Jan-Feb:5(1):17-22. doi: 10.1097/APO.0000000000000178. Epub
[PubMed PMID: 26886115]
[92]
Zaharia AC, Dumitrescu OM, Radu M, Rogoz RE. Adherence to Therapy in Glaucoma Treatment-A Review. Journal of personalized medicine. 2022 Mar 22:12(4):. doi: 10.3390/jpm12040514. Epub 2022 Mar 22
[PubMed PMID: 35455630]
[93]
Holló G, Katsanos A, Boboridis KG, Irkec M, Konstas AGP. Preservative-Free Prostaglandin Analogs and Prostaglandin/Timolol Fixed Combinations in the Treatment of Glaucoma: Efficacy, Safety and Potential Advantages. Drugs. 2018 Jan:78(1):39-64. doi: 10.1007/s40265-017-0843-9. Epub
[PubMed PMID: 29196953]
[94]
Oh DJ, Chen JL, Vajaranant TS, Dikopf MS. Brimonidine tartrate for the treatment of glaucoma. Expert opinion on pharmacotherapy. 2019 Jan:20(1):115-122. doi: 10.1080/14656566.2018.1544241. Epub 2018 Nov 8
[PubMed PMID: 30407890]
Level 3 (low-level) evidence
[95]
Skov AG, Rives AS, Freiberg J, Virgili G, Azuara-Blanco A, Kolko M. Comparative efficacy and safety of preserved versus preservative-free beta-blockers in patients with glaucoma or ocular hypertension: a systematic review. Acta ophthalmologica. 2022 May:100(3):253-261. doi: 10.1111/aos.14926. Epub 2021 Jun 14
[PubMed PMID: 34128326]
Level 1 (high-level) evidence
[96]
Supuran CT, Altamimi ASA, Carta F. Carbonic anhydrase inhibition and the management of glaucoma: a literature and patent review 2013-2019. Expert opinion on therapeutic patents. 2019 Oct:29(10):781-792. doi: 10.1080/13543776.2019.1679117. Epub 2019 Oct 15
[PubMed PMID: 31596641]
Level 3 (low-level) evidence
[98]
Greslechner R, Spiegel D. [Laser Trabeculoplasty in Modern Glaucoma Therapy - a Review]. Klinische Monatsblatter fur Augenheilkunde. 2019 Oct:236(10):1192-1200. doi: 10.1055/a-0577-7925. Epub 2018 Apr 11
[PubMed PMID: 29642265]
[99]
Landers J. Selective laser trabeculoplasty: A review. Clinical & experimental ophthalmology. 2021 Dec:49(9):1102-1110. doi: 10.1111/ceo.13979. Epub 2021 Aug 16
[PubMed PMID: 34331388]
[100]
Ma A, Yu SWY, Wong JKW. Micropulse laser for the treatment of glaucoma: A literature review. Survey of ophthalmology. 2019 Jul-Aug:64(4):486-497. doi: 10.1016/j.survophthal.2019.01.001. Epub 2019 Jan 11
[PubMed PMID: 30639207]
Level 3 (low-level) evidence
[101]
Souissi S, Le Mer Y, Metge F, Portmann A, Baudouin C, Labbé A, Hamard P. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta ophthalmologica. 2021 Aug:99(5):e621-e653. doi: 10.1111/aos.14661. Epub 2020 Nov 22
[PubMed PMID: 33222409]
[102]
Lim R. The surgical management of glaucoma: A review. Clinical & experimental ophthalmology. 2022 Mar:50(2):213-231. doi: 10.1111/ceo.14028. Epub 2022 Jan 17
[PubMed PMID: 35037376]
[103]
King AJ, Hudson J, Fernie G, Kernohan A, Azuara-Blanco A, Burr J, Homer T, Shabaninejad H, Sparrow JM, Garway-Heath D, Barton K, Norrie J, McDonald A, Vale L, MacLennan G, TAGS Study Group. Primary trabeculectomy for advanced glaucoma: pragmatic multicentre randomised controlled trial (TAGS). BMJ (Clinical research ed.). 2021 May 12:373():n1014. doi: 10.1136/bmj.n1014. Epub 2021 May 12
[PubMed PMID: 33980505]
Level 1 (high-level) evidence
[104]
Muralidharan S, Kumar S, Ichhpujani P, Dhillon HK. Quality of life in glaucoma patients: Comparison of medical therapy, trabeculectomy, and glaucoma drainage device surgery. Indian journal of ophthalmology. 2022 Dec:70(12):4206-4211. doi: 10.4103/ijo.IJO_667_22. Epub
[PubMed PMID: 36453315]
Level 2 (mid-level) evidence
[105]
Wong D. Non-penetrating glaucoma surgery for advanced open-angle glaucoma. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018 Aug:256(8):1479. doi: 10.1007/s00417-018-4026-5. Epub 2018 Jun 18
[PubMed PMID: 29911272]
[106]
Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clinical ophthalmology (Auckland, N.Z.). 2016:10():189-206. doi: 10.2147/OPTH.S80490. Epub 2016 Jan 28
[PubMed PMID: 26869753]
[107]
Pereira ICF, van de Wijdeven R, Wyss HM, Beckers HJM, den Toonder JMJ. Conventional glaucoma implants and the new MIGS devices: a comprehensive review of current options and future directions. Eye (London, England). 2021 Dec:35(12):3202-3221. doi: 10.1038/s41433-021-01595-x. Epub 2021 Jun 14
[PubMed PMID: 34127842]
Level 3 (low-level) evidence
[108]
Miljković A, Babić N, Čanadanović V, Davidović S, Ljikar J, Vasin M. EFFICACY OF CYCLOCRYOTHERAPY AND TRANSSCLERAL DIODE LASER CYCLOPHOTOCOAGULATION IN THE MANAGEMENT OF REFRACTORY GLAUCOMA. Acta clinica Croatica. 2021 Jun:60(2):171-177. doi: 10.20471/acc.2021.60.02.01. Epub
[PubMed PMID: 34744265]
[109]
Kowanz DH, Wawer Matos PA, Gordon E, Doulis A, Simon M, Rokohl AC, Heindl LM. [Evisceration, enucleation and exenteration-Indications, techniques, and postoperative care]. Die Ophthalmologie. 2023 Feb:120(2):126-138. doi: 10.1007/s00347-022-01791-4. Epub 2023 Jan 12
[PubMed PMID: 36635593]
[110]
Galindo-Ferreiro A, Akaishi P, Cruz A, Khandekar R, Al Dosairi S, Dufaileej M, Al Salem A, Galvez-Ruiz A, Craven ER. Retrobulbar Injections for Blind Painful Eyes: A Comparative Study of Retrobulbar Alcohol Versus Chlorpromazine. Journal of glaucoma. 2016 Nov:25(11):886-890
[PubMed PMID: 27814327]
Level 2 (mid-level) evidence
[112]
Brusini P. Estimating glaucomatous anatomical damage by computerized automated perimetry. Acta ophthalmologica Scandinavica. Supplement. 1997:(224):28-9
[PubMed PMID: 9589720]
[113]
Spaeth GL, Lopes JF, Junk AK, Grigorian AP, Henderer J. Systems for staging the amount of optic nerve damage in glaucoma: a critical review and new material. Survey of ophthalmology. 2006 Jul-Aug:51(4):293-315
[PubMed PMID: 16818081]
Level 3 (low-level) evidence
[114]
Brusini P. OCT Glaucoma Staging System: a new method for retinal nerve fiber layer damage classification using spectral-domain OCT. Eye (London, England). 2018 Jan:32(1):113-119. doi: 10.1038/eye.2017.159. Epub 2017 Aug 4
[PubMed PMID: 28776589]
[115]
Ng M, Sample PA, Pascual JP, Zangwill LM, Girkin CA, Liebmann JM, Weinreb RN, Racette L. Comparison of visual field severity classification systems for glaucoma. Journal of glaucoma. 2012 Oct-Nov:21(8):551-61. doi: 10.1097/IJG.0b013e31821dac66. Epub
[PubMed PMID: 21878817]
[116]
Wilson MR. Progression of visual field loss in untreated glaucoma patients and suspects in St Lucia, West Indies. Transactions of the American Ophthalmological Society. 2002:100():365-410
[PubMed PMID: 12545702]
[117]
Mokhles P, Schouten JS, Beckers HJ, Azuara-Blanco A, Tuulonen A, Webers CA. Glaucoma blindness at the end of life. Acta ophthalmologica. 2017 Feb:95(1):10-11. doi: 10.1111/aos.12933. Epub
[PubMed PMID: 28102642]
[118]
Fiscella R, Caplan E, Kamble P, Bunniran S, Uribe C, Chandwani H. The Effect of an Educational Intervention on Adherence to Intraocular Pressure-Lowering Medications in a Large Cohort of Older Adults with Glaucoma. Journal of managed care & specialty pharmacy. 2018 Dec:24(12):1284-1294. doi: 10.18553/jmcp.2018.17465. Epub 2018 May 31
[PubMed PMID: 29848186]
[119]
Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian journal of ophthalmology. 2011 Jan:59 Suppl(Suppl1):S93-6. doi: 10.4103/0301-4738.73693. Epub
[PubMed PMID: 21150041]
[120]
Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009 Nov:116(11 Suppl):S30-6. doi: 10.1016/j.ophtha.2009.06.024. Epub
[PubMed PMID: 19837258]
Level 3 (low-level) evidence
[121]
Friedman DS, Quigley HA, Gelb L, Tan J, Margolis J, Shah SN, Kim EE, Zimmerman T, Hahn SR. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Investigative ophthalmology & visual science. 2007 Nov:48(11):5052-7
[PubMed PMID: 17962457]
[122]
Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005 Jun:112(6):953-61
[PubMed PMID: 15885795]
[123]
Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005 Aug 4:353(5):487-97
[PubMed PMID: 16079372]