Continuing Education Activity
Dermatofibrosarcoma protuberans (DFSP) is an uncommon soft tissue tumor that involves the dermis, subcutaneous fat, and in rare cases, muscle and fascia. The tumor typically presents as a slowly growing, firm plaque on the trunk of young adults. The cause of dermatofibrosarcoma protuberans is not clearly understood, though studies have implicated a chromosomal translocation that results in a fusion protein that promotes tumor growth through the overproduction of platelet-derived growth factor (PDGF). Diagnosis is made via skin biopsy. This activity describes the evaluation, diagnosis, and management of dermatofibrosarcoma protuberans and stresses the role of team-based interprofessional care for affected patients.
Objectives:
Describe the population most at risk for developing dermatofibrosarcoma protuberans.
Review the typical clinical presentation of a patient with dermatofibrosarcoma protuberans.
Outline the treatment strategy for a patient with dermatofibrosarcoma protuberans.
Summarize interprofessional team strategies for improving care coordination and communication to enhance outcomes for patients affected by dermatofibrosarcoma protuberans.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare soft tissue tumor that involves the dermis, subcutaneous fat, and in rare cases, muscle and fascia. The tumor typically presents as a slowly growing, firm plaque on the trunk of young adults. The cause of dermatofibrosarcoma protuberans is not clearly understood. Studies have implicated a chromosomal translocation, resulting in the fusion protein COL1A1-PDGFB, which promotes tumor growth through the overproduction of platelet-derived growth factor (PDGF). Diagnosis is made via skin biopsy. Dermatofibrosarcoma protuberans is considered an intermediate-grade malignancy with a low likelihood of metastasis but a high local recurrence rate. Given its propensity for a subclinical extension, the optimal treatment modality for dermatofibrosarcoma protuberans is Mohs micrographic surgery (MMS), a surgical technique that allows complete margin assessment and tissue preservation. Alternatively, dermatofibrosarcoma protuberans can be treated with wide local excision. The chemotherapeutic agent imatinib mesylate is currently FDA-approved for adults with unresectable, recurrent, or metastatic dermatofibrosarcoma protuberans.[1][2][3][4][5]
Etiology
The mesenchymal tumor is thought to originate from a dermal stem cell or undifferentiated mesenchymal cell with fibroblastic, muscular, and neurologic features. The cause of dermatofibrosarcoma protuberans unknown, though a proposed risk factor includes injury to the skin in the affected location. Occurrences within preexisting scars and tattoos have been reported.
Epidemiology
Dermatofibrosarcoma protuberans is a rare tumor with an incidence rate of 0.8 to 4.5 cases per million persons per year. It accounts for between 1% and 6% of all soft tissue sarcomas and 18% of all cutaneous soft tissue sarcomas. The pigmented variant, known as a Bednar tumor, and the fibrosarcomatous variant account for less than 5% and 5% to 15% of all dermatofibrosarcoma protuberans cases, respectively. Dermatofibrosarcoma protuberans occurs most often in adults in the third to fifth decades of life but has been reported in all age groups, including congenital presentations. Studies disagree as to whether there is a slight female or male predominance. During pregnancy, dermatofibrosarcoma protuberans can exhibit accelerated growth. Dermatofibrosarcoma protuberans, particularly Bednar tumors, occur most frequently in black patients.
Pathophysiology
Studies have shown that dermatofibrosarcoma protuberans demonstrates the chromosomal translocation t(17;22)(q22;q13) between chromosome 17 and chromosome 22, which is thought to be key in the tumor’s pathogenesis. The chromosomal translocation leads to the fusion of platelet-derived growth factor-beta polypeptide gene (PDGFB) and collagen type 1A1 gene (COL1A1). The gene rearrangement upregulates PDGFB, resulting in overproduction of PDGF, continuous autocrine activation of platelet-derived growth factor receptor-beta (PDGFRb), cellular proliferation, and tumor formation. This chromosomal translocation is present in over 90% of dermatofibrosarcoma protuberans. In cases without the COL1A1/PDGFB translocation, a different chromosomal translocation still involving PDGFB on chromosome 22 has been demonstrated.
Histopathology
DFSP is poorly circumscribed and usually involves dermis and subcutis. Rare cases can be limited to the dermis. DFSP is considered an intermediate tumor between a benign dermatofibroma and a frank fibrosarcoma due to the possibility of having distant metastasis and aggressive local invasion. Transformation to a high-grade sarcoma is extremely rare.
The overlying epidermis does not show any atypical histological features, but macroscopically the skin appears greyish or lightly pigmented.
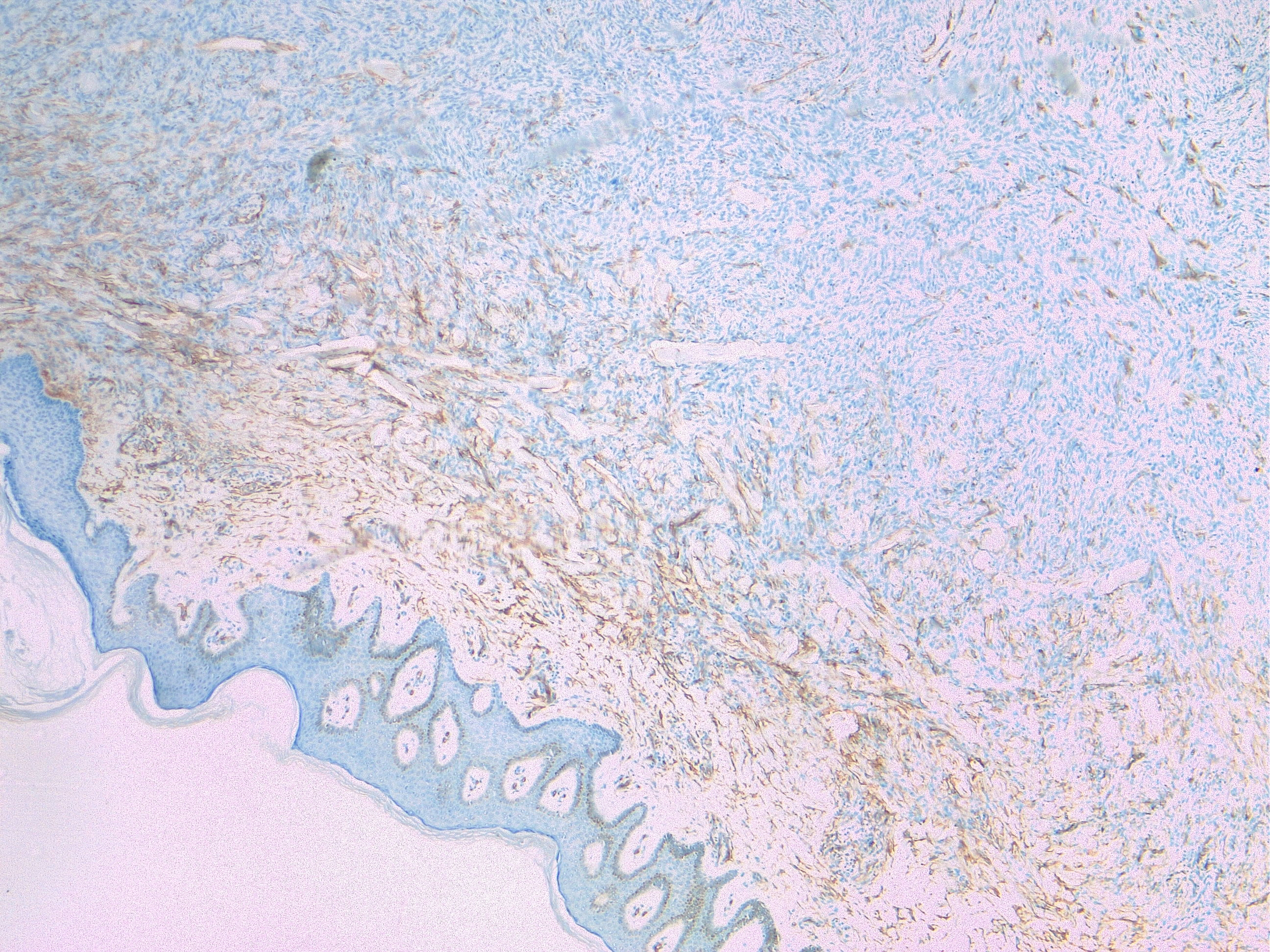
Tumor cells are arranged in a storiform or intersecting pattern, parallel to the epidermal surface. DFSP is constituted by spindle cells with little pleomorphism and scant cytoplasm. Mitoses are present, but there is no striking mitotic activity, and atypical mitosis is rare. A mitotic count that reaches 10/10 HPF and tumor size correlates with metastatic spread [6]. The cells are surrounded by collagenous stroma, sometimes associated with hyaline or myxoid changes. Infiltration to the underlying tissue (subcutis, fascia, muscle, periosteum) is a common feature, usually with neoplastic tentacles that shape a characteristic honeycomb appearance. Necrosis is uncommon. Fibrosarcomatous transformation is seen as an expansive tumor with fascicular and herringbone pattern, atypical cytological features; it can happen in about a fifth of all DFSP, ranging 10 to 20%, arising in all histologic subtypes.An immunohistochemical panel is useful for diagnosing DFSP. CD34 is commonly positive (80–100% of cases), while FXIIIa, SMA, desmin, S100, and keratins are negative.
All DFSP show a recurrent translocation: t(17;22)(q22;q13) resulting in COL1A1-PDGFB fusion. This constitutively activates the tyrosine kinase PDGF-B receptor and leads to altered cell cycle control and tumor spread.
Differential diagnosis of DFSP include:
- Cellular fibrous histiocytoma/dermatofibroma, negative for CD34 and positive for FXIIIa;
- Solitary fibrous tumor: CD34 and STAT-6 positive, has a typical NAB2-STAT6 gene fusion;
- Spindle cell lipoma: S100 positive
- Angiosarcoma: positive for CD31 and ERG
- Peripheral nerve sheath tumors that express S100, SOX-10, GFAP;
- Spindle cell melanoma that expresses S100, MART1/MELANA
- Angiomyxoma,
- Myxoid sarcoma
- Synovial sarcoma is rarely positive for CD34 but is positive for TLE1 and EMA.
- Sarcomatoid carcinoma: pleomorphic variant of undifferentiated carcinomas and is CD34-negative, keratin-positive.
History and Physical
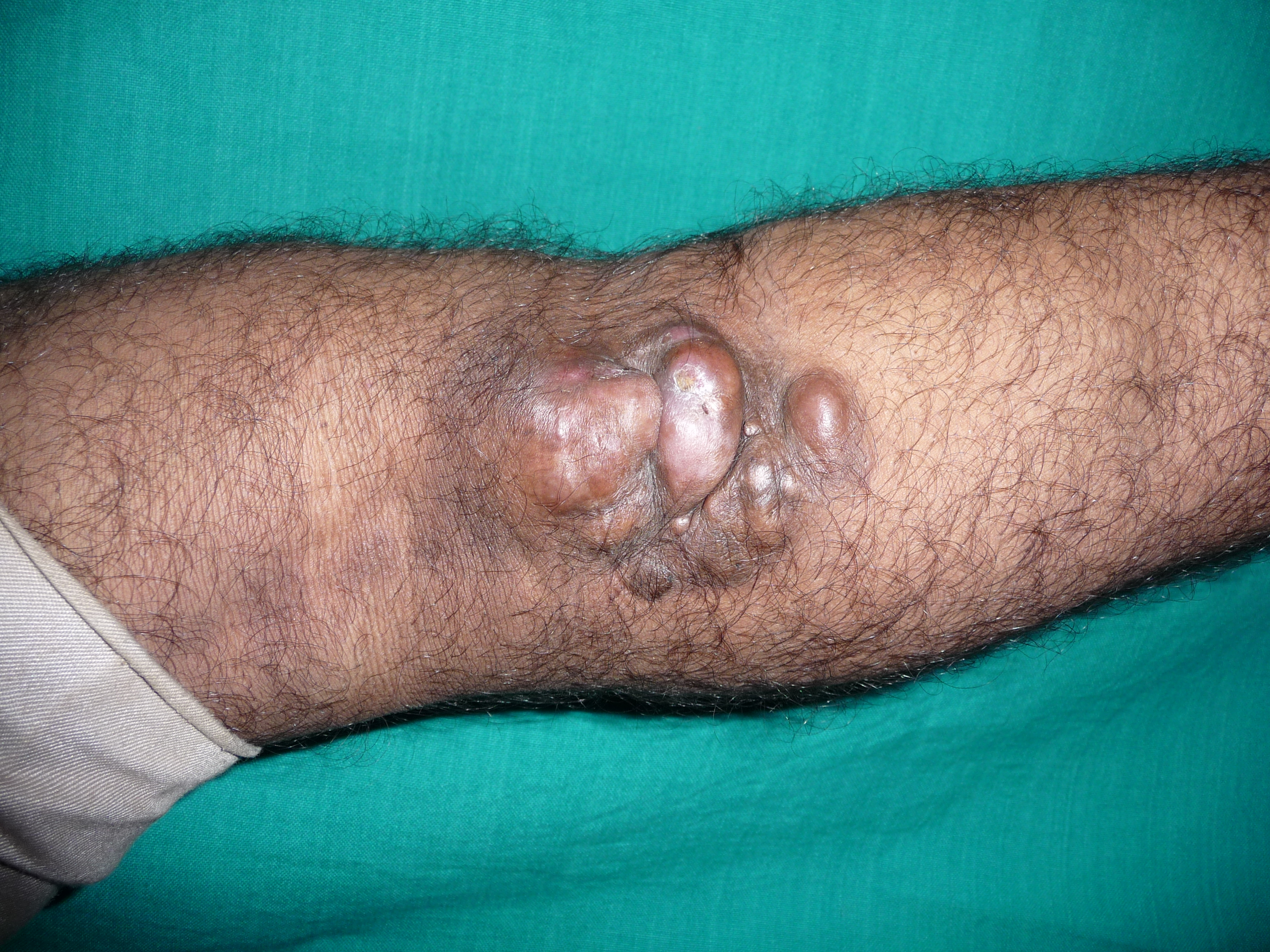
Dermatofibrosarcoma protuberans typically presents as an asymptomatic, skin-colored to red-brown indurated plaque, which eventually develops multiple raised violaceus to red-brown nodules. They grow slowly and can reach several centimeters in diameter. The atrophic variant presents as a violaceous plaque that may resemble morphea or scar. The tumor, particularly in the early stages, can resemble a keloid or dermatofibroma and is often misdiagnosed. As they grow larger, some can ulcerate and become painful. The majority of DFSPs occur on the trunk (50%), followed by the extremities (35%) and then head and neck (15%).
The shoulder and pelvic region are particularly characteristic areas for dermatofibrosarcoma protuberans. Dermatofibrosarcoma protuberans usually extends into the subcutaneous fat but rarely involves the fascia, muscle, or bone unless it is longstanding or recurrent. Dermatofibrosarcoma protuberans is known to have relentless growth with asymmetric, root-like projections which cannot be appreciated on clinical exam. Thus, local recurrence following excision is common. Distant metastasis is rare, occurring in 1% to 4% of cases and usually only after multiple local recurrences. The lung is the most common site of metastasis via hematogenous spread. The regional lymph nodes are rarely involved. The fibrosarcomatous variant of dermatofibrosarcoma protuberans has a higher risk of local recurrence (14% to 52%) and distant metastases (8% to 29%).
Evaluation
Diagnosis of dermatofibrosarcoma protuberans is made with a skin biopsy, preferably an incisional or excisional biopsy. A full history and physical exam, including lymph node examination, should be completed. Some sources suggest obtaining chest imaging to evaluate for any metastatic disease before treatment, though this is not currently a general recommendation. A preoperative MRI, though not necessary, is sometimes performed to help define tumor extension before surgery.[7][8][9][10]
Treatment / Management
The treatment of dermatofibrosarcoma protuberans is surgical removal, ideally with Mohs micrographic surgery (MMS), a surgical technique that ensures complete histopathologic margin control at the time of surgery. MMS is preferred over wide local excision as dermatofibrosarcoma protuberans tends to have an unpredictable subclinical extension. In select situations or when Mohs micrographic surgery is not available, wide local excision may be performed with 2- to 4 cm margins. However, local recurrence is relatively common with wide local excision even with clear surgical margins. Dermatofibrosarcoma protuberans treated with wide local excision has a recurrence rate of about 7.3% compared to a recurrence rate of about 1% when treated with MMS. If feasible, modified wide local excision with horizontal sectioning, as opposed to the usual histologic vertical processing (‘bread-loafing”), and detailed mapping can be performed to decrease the likelihood of local recurrence. As spread to the lymph nodes is extremely rare, regional node dissection is not recommended.
The chemotherapy agent imatinib mesylate, an oral tyrosine kinase inhibitor, can be used for recurrent, unresectable, and metastatic dermatofibrosarcoma protuberans in adults. Imatinib mesylate competitively inhibits ATP binding to the PDGF-beta receptor, a tyrosine kinase. This slows down kinase activity, limiting the growth of the tumor, and promoting apoptosis. Patients with the t(17;22) translocation show a greater response to imatinib mesylate, and thus screening for this translocation should be performed before initiating therapy. Testing for the translocation can be performed using fluorescent in situ hybridization (FISH) or reverse transcription-polymerase chain reaction (RT-PCR). Side effects of imatinib mesylate include gastrointestinal upset, edema, fatigue, anemia, and rash. The majority of patients with dermatofibrosarcoma protuberans with the translocation respond favorably to imatinib mesylate therapy, with studies suggesting a response rate of approximately 65%. The duration of therapy varies. Some sources recommend 6 months of therapy, but this may be extended if needed. Alternatively, radiation therapy may also be used for unresectable or recurrent tumors, and adjuvant radiation may decrease the risk of local recurrence.[11][12]
As local recurrence is common, patients require close clinical follow-up after completing treatment. The risk of recurrence is highest in the first 3 years after treatment, and thus patients should be evaluated every 3 to 6 months during this time and at least annually thereafter. Some sources advocate baseline and serial chest CT scans, especially in the case of fibrosarcomatous dermatofibrosarcoma protuberans, which has a higher risk of local recurrence and metastasis. Otherwise, routine imaging is not required unless there are symptoms to suggest metastasis.
Differential Diagnosis
- Cutaneous melanoma
- Dermatofibroma
- Dermatologic metastatic carcinoma
- Epidermal inclusion cyst
- Keloid scar
- Morphea
Prognosis
The overall prognosis of dermatofibrosarcoma protuberans is good, with a 10-year survival rate of 99.1%. As metastasis is rare, morbidity due to local recurrence is a more common issue. Age older than 50 is a risk factor for local recurrence. Patients with metastatic disease live on average about 2 years after the time of diagnosis. A high mitotic index, increased cellularity, black race, male sex, and location on the head, neck, or limb are risk factors for higher mortality rates.
Complications
While the prognosis for dermatofibrosarcoma protuberans is quite good, the main complication is the rare instances where it can metastasize. Other complications center around post-surgical scarring and cosmesis.
Deterrence and Patient Education
Patients must understand that post-treatment, continued visits to a dermatologist are crucial. When dermatofibrosarcoma protuberans returns, it is usually within 3 years; they should schedule an examination quarterly until that time. Afterward, assuming no recurrence, annual visits are sufficient.[13]
Pearls and Other Issues
In cases of advanced or metastatic dermatofibrosarcoma protuberans, coordinated care with multiple specialties is crucial. A dermatologic surgeon, ideally a Mohs micrographic surgeon, should be involved in the surgical treatment of dermatofibrosarcoma protuberans.
Enhancing Healthcare Team Outcomes
The diagnosis and management of dermatofibrosarcoma protuberans require an interprofessional team that consists of an oncologist, surgeon, radiologist, radiation therapist, and physical therapy. The treatment of dermatofibrosarcoma protuberans is surgical removal. The key is to remove the entire lesion with clear margins. Local recurrences are very common when margins are positive. Dermatofibrosarcoma protuberans does not respond well to chemotherapy or radiation. All patients need long-term follow-up with imaging studies because recurrences are known to occur. The prognosis for patients with completely excised lesions is good.[14] [Level 5]