Introduction
The rectum initiates the terminal section of the large intestine at the level of the S3 vertebra, just after the sigmoid colon. Approximately 15 cm long, it is characterized by the cessation of the omental appendices and the absence of teniae coli and haustra. It is distally continuous with the anal canal as it protrudes through the pelvic diaphragm/levator ani. The rectum plays a significant role in fecal continence and the storage of feces via the ampulla. The anal canal is the final portion of the large intestine and is approximately 3 cm long. The anal canal also contributes to the maintenance of fecal continence and has great clinical significance due to the different embryological origins of the superior and inferior sections of the canal. Disruption in the development of the hindgut either environmentally or genetically can cause many disorders of the rectum and anal canal.[1][2]
Development
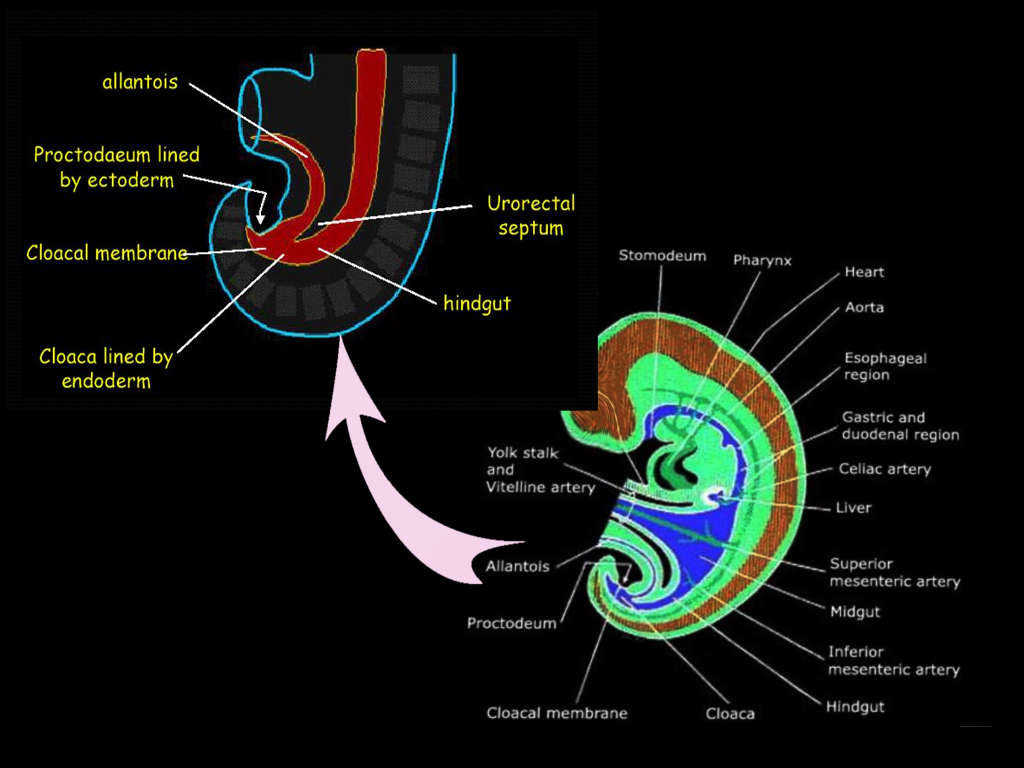
The gastrointestinal tract arises by the beginning of the third week of embryonic development. During a process called gastrulation, the three germ layers form. The germ layers include the ectoderm, mesoderm, and endoderm, which together compose the embryonic disc. The cranial end of the embryonic disc consists of the oropharyngeal membrane, which ultimately forms the mouth, and the caudal end includes the cloacal membrane, which forms the anus.[3] During the fourth week, the primordial gastrointestinal tract begins to form from the endoderm. As the embryo folds towards the midline, the endoderm creates a gut tube that is suspended by mesentery, or double layer of peritoneum. The gut tube divides into the foregut, midgut, and hindgut. The hindgut forms the distal one-third of the transverse colon, the descending colon, the sigmoid colon, rectum, and the superior portion of the anal canal. The terminal end of the hindgut includes the cloaca, which forms not only the gastrointestinal tract but also the urogenital tract.
Towards the caudal end of the cloaca is an area called the cloacal membrane, which is a boundary between the endoderm layer and the ectoderm layer with no mesoderm in between; this ultimately forms the proctodeum. A mesoderm-derived urorectal septum divides the cloaca into the ventral urogenital cavity and the dorsal anorectal canal by week seven or eight.[3] At this point, the terminal ends of both the urogenital cavity and the anorectal canal open at the cloacal membrane. This opening at the cloacal membrane is significant because the anal canal now forms from both endoderm and ectoderm. The superior two-thirds of the anal canal derives from the hindgut, and the inferior one-third of the anal canal derives from the ectodermal proctodeum. The junction that delineates these two epithelia is called the pectinate line or the dentate line.[3]
Function
The rectum plays a role in fecal continence and the storage of feces. Directly above the pelvic diaphragm or the levator ani and the anococcygeal ligament is a dilation of the terminal rectum known as the ampulla. This area is the location where feces accumulate and remain until defecation. The ampulla of the rectum is very distensible and is capable of regulating the movement of fecal material into the anal canal for expulsion. The shape of the rectum, in conjunction with the flexures created along the way, also aids in the mechanism of fecal continence.[14]
The anal canal also helps with the storage and expulsion of feces. The superior portion of the anal canal contains a circular muscle layer called the internal anal sphincter, which is tonically contracted most of the time to prevent leakage. The involuntary contraction of this muscle gets stimulated by the sympathetic nervous system and gets inhibited by the parasympathetic nervous system. As feces or gas passes through, the distension in the rectal ampulla causes a temporary relaxation of the internal anal sphincter and a voluntary contraction of the external anal sphincter, which is in the inferior portion of the anal canal.[15] Occasionally, recruitment of the puborectalis muscle is required to halt fecal expulsion. For defecation to occur, both sphincters must be relaxed.[14]
Testing
Anorectal manometry: Measures and assesses the anal sphincter (internal and external) and rectal pressure and its function. This method is used to evaluate patients with fecal incontinence and constipation. It can directly measure the luminal pressure, including the high-pressure zone, resting pressure, squeezing pressure, rectal sensation/compliance, and the anorectal inhibitory reflex.[16]
Defecating proctography/Defecography: A study using X-ray imaging to evaluate anatomic defects of the anorectal region and function of the puborectalis muscle. A contrast filled paste gets initially introduced to the rectum, and the patient is instructed to defecate in a series of stages (relaxation, contraction, tensing of the abdomen, and evacuation).[16]
Balloon capacity and compliance test: Evaluates the function of the rectum using a device (plastic catheter with a latex balloon attached), which is inserted into the rectum and gradually filled with warm water. During this process, the volume and pressure are measured.[16]
Balloon evacuation study: This test is similar to the balloon capacity and compliance test in which a catheter with a small balloon gets inserted into the rectum and filled with water. Different volumes of water get loaded inside the balloon, and the patient is instructed to evacuate the balloon. This procedure is done to evaluate the opening of the anal canal and to assess the relaxation of the pelvic floor.[16]
Pudendal nerve terminal motor latency: A probe designed to stimulate and record nerve activity is placed on the physician's gloved finger, which is then inserted into the rectum to measure pudendal nerve activity (latency to contraction of the anal sphincter muscle). The pudendal nerve innervates the anal sphincter muscles; therefore, this test can be used to assess any injury to that nerve.[16]
Electromyography: A test to measure the ability of the puborectalis muscle and sphincter muscles to relax properly. An electrode is placed inside the rectum, and the activity of these muscles gets evaluated throughout a series of stages (relaxation, contraction, and evacuation).[16]
Endoanal Ultrasonography: The use of ultrasound imaging to examine rectal lesions, defects, or injuries to the surrounding tissues.[16]
Suction rectal biopsy: Gold standard for the diagnosis of Hirschsprung disease. A biopsy is taken two cm above the dentate line, and the absence of ganglion cells on histology confirms the diagnosis. Hypertrophic nerve fibers may be present in addition to this finding.[16]
Contrast enema: Used as one of the diagnostic methods for Hirschsprung disease. Useful for localization of the aganglionic segment by looking for a narrowed rectum. Diagnostic confirmation is via a rectal biopsy.[16]
Pathophysiology
The pectinate or dentate line is the junction between the superior and inferior anal canal. There are many differences between these two regions, including their embryological origins, innervation, venous and arterial supply, and lymphatic supply. Above the pectinate line, the anal canal has an endodermal origin and is lined by simple columnar epithelia. Blood supply is from the superior rectal artery, which originates from the inferior mesenteric artery and returns via the superior rectal veins into the inferior mesenteric vein.[17] Due to the venous anastomoses that occurs in the anal canal and the backup of blood flow into the rectal veins, hemorrhoids may be present in patients with portal hypertension. The lymphatic drainage of the superior anal canal is via internal iliac lymph nodes. The superior anal canal receives innervation from the inferior hypogastric plexus, which is a visceral innervation. It has both sympathetic and parasympathetic functions that control the tonicity of the internal anal sphincter, thereby contributing to the rectal ampulla reflex. The rectal ampulla senses the distension created by the buildup of feces and causes an inhibitory reflex of the internal anal sphincter, facilitating fecal continence.
Below the pectinate line, the anal canal has an ectodermal origin and is predominantly lined by stratified squamous epithelium. The inferior rectal canal obtains its blood supply from the inferior rectal artery, which originates from the internal iliac artery. Blood returns via the inferior rectal vein, which ultimately drains into the inferior vena cava.[17] The lymphatic drainage of the inferior anal canal is the superficial inguinal lymph nodes. The inferior anal canal receives somatic innervation via the branches of the pudendal nerve, specifically the inferior anal/rectal nerve. Its efferent somatic innervation controls the voluntary actions of the external anal sphincter. Due to its somatic innervation, it can sense pain, temperature, and touch. This difference in innervation is implicated in the clinical presentation of hemorrhoids and anal fissures. Patients with lesions below the pectinate line usually complain of significant pain with any contact near the lesions, and pain is often unbearable, whereas lesions above the pectinate line often go unnoticed because of its visceral innervation and the lack of pain sensation.[2]