Continuing Education Activity
Intracranial hemorrhage comprises 4 broad types of hemorrhage, including epidural hemorrhage, subdural hemorrhage, subarachnoid hemorrhage, and intraparenchymal hemorrhage. The first 2 types of hemorrhages—epidural and subdural—are referred to as extra-axial hemorrhages, whereas the latter 2 hemorrhages—subarachnoid and intraparenchymal—are categorized as intra-axial. Each type of hemorrhage arises from distinct etiologies, leading to variable clinical findings, prognosis, and outcomes.
Immediate neurosurgical consultation is strongly advised for all cases of hemorrhage, especially when alarming signs such as airway compromise, respiratory failure, or hemodynamic instability are evident. Proper evaluation, imaging, and monitoring for signs of impending deterioration should guide the implementation of best practice management. Early neurosurgical consultation is essential, as management strategies vary depending on the type of hemorrhage. This activity offers a comprehensive overview of intracranial hemorrhage types and the crucial role of the interprofessional healthcare team in treating patients experiencing various types of intracranial hemorrhages. This activity also enables clinicians to gain insights into integrating specialist consultation with evidence-based clinical judgment to mitigate the risk of morbidity and mortality associated with intracranial hemorrhages.
Objectives:
Identify signs and symptoms indicative of intracranial hemorrhage in patients of varying age groups and demographics.
Implement evidence-based protocols and proper evaluation, imaging, and monitoring protocols for timely diagnosis and management of intracranial hemorrhage.
Select appropriate specialist consultations, including neurology, neurosurgery, and radiology, as part of the interprofessional healthcare team approach to optimize patient care and outcomes.
Coordinate care seamlessly among different specialties of an interprofessional healthcare team to ensure comprehensive management of intracranial hemorrhage cases, leading to enhanced outcomes.
Introduction
Intracranial hemorrhage encompasses 4 broad types of hemorrhage[1][2][3]:
- Epidural hemorrhage
- Subdural hemorrhage
- Subarachnoid hemorrhage (SAH)
- Intraparenchymal hemorrhage: A hemorrhage involving the cerebral hemispheres.
The first two are called extra-axial hemorrhage, and the latter are intra-axial. Each type of hemorrhage differs concerning etiology, findings, prognosis, and outcome. This article provides a broad overview of the types of intracranial hemorrhage.
Etiology
Epidural Hemorrhage
An epidural hematoma is arterial or venous in origin. The classical arterial epidural hematoma occurs after blunt trauma to the head, typically the temporal region. This type of bleeding may also occur after a penetrating head injury. Typically, a skull fracture with damage to the middle meningeal artery causes arterial bleeding into the potential epidural space. Although the middle meningeal artery is classically described, any meningeal artery can lead to arterial epidural hematoma.[4]
A venous epidural hematoma occurs after a skull fracture, and the venous bleeding from the skull fracture fills the epidural space. Venous epidural hematomas are common in pediatric patients.
Subdural Hemorrhage
Subdural hemorrhage occurs when blood enters the subdural space, which is anatomically the arachnoid space. Commonly, subdural hemorrhage occurs after a vessel traversing between the brain and skull is stretched, broken, or torn and begins to bleed into the subdural space. These most commonly occur after a blunt head injury but may also occur after penetrating head injuries or spontaneously.[5][6]
Subarachnoid Hemorrhage
A subarachnoid hemorrhage refers to bleeding into the subarachnoid space. Subarachnoid hemorrhage is divided into traumatic versus non-traumatic subarachnoid hemorrhage. A second categorization divides subarachnoid hemorrhage into an aneurysmal and non-aneurysmal subarachnoid hemorrhage. Aneurysmal subarachnoid hemorrhage occurs after the rupture of a cerebral aneurysm. Non-aneurysmal subarachnoid hemorrhage is bleeding into the subarachnoid space without identifiable aneurysms. Non-aneurysmal subarachnoid bleeding most commonly occurs after trauma with a blunt head injury with or without penetrating trauma or sudden acceleration changes to the head.[7]
Intraparenchymal Hemorrhage
Intraparenchymal hemorrhage is bleeding into the brain parenchyma. A wide variety of reasons cause hemorrhage, including, but not limited to, hypertension, arteriovenous malformation (AVM), amyloid angiopathy, aneurysm rupture, tumor, coagulopathy, infection, vasculitis, and trauma.
Epidemiology
Epidural Hemorrhage
Epidural hematomas are present in approximately 2% of head injury patients and account for 5% to 15% of fatal head injuries. Approximately 85% to 95% of epidural hematomas have an overlying skull fracture. 3.1 million people worldwide require surgery for traumatic epidural hemorrhage every year.[8]
Subdural Hemorrhage
The incidence of subdural hematoma is estimated to be between 5% and 25% of patients with a significant head injury. It is present in an annual incidence of 1 to 5 cases per 100,000 per year, with a male-to-female ratio of 2:1. The incidence of subdural hematomas increases throughout life.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage accounts for approximately 5% of all strokes and has an incidence of approximately 2 to 25 per 100,000 person-years for those over the age of 35. The incidence trends slowly as patients age and may be more frequent in females than males (1.15:1 for the female-to-male ratio).
Intraparenchymal Hemorrhage
Intraparenchymal hemorrhage accounts for 10% to 20% of all strokes. Intraparenchymal hemorrhage incidence increases for those aged 55 and older, with an increasing incidence as age increases. Some controversy exists regarding gender differences, with a slight male predominance.
Pathophysiology
Epidural Hemorrhage
Epidural hematomas occur when blood dissects into the potential space between the dura and the inner table of the skull. Most commonly, this happens after a skull fracture (85% to 95% of cases). Damage to an arterial or venous vessel may occur, which causes blood to dissect into the potential epidural space, resulting in the epidural hematoma. The most commonly damaged vessel is the middle meningeal artery underlying the temporoparietal region of the skull.
Subdural Hemorrhage
Subdural hematoma has multiple causes, including head trauma, coagulopathy, vascular abnormality rupture, and spontaneous. Most commonly, head trauma causes motion of the brain relative to the skull, which can stretch and break blood vessels traversing from the brain to the skull. If the blood vessels are damaged, they bleed into the subdural space. Chronic subdural hematoma usually results secondary to trauma that results in bleeding from bridging veins. Subsequent neovascularization and leaking from the outer membrane result in the expansion of the hematoma.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage most commonly occurs after trauma, where cortical surface vessels are injured and bleed into the subarachnoid space. Non-traumatic subarachnoid hemorrhage is most commonly due to the rupture of a cerebral aneurysm. When an aneurysm ruptures, blood can flow into the subarachnoid space. Other causes of subarachnoid hemorrhage include AVM, use of blood thinners, trauma, or idiopathic causes.
Intraparenchymal Hemorrhage
Non-traumatic intraparenchymal hemorrhage often occurs secondary to hypertensive damage to cerebral blood vessels, which eventually burst and bleed into the brain. Other causes include rupture of an AVM, rupture of an aneurysm, arteriopathy, tumor, infection, or venous outflow obstruction. Penetrating and non-penetrating trauma may also cause intraparenchymal hemorrhage. The most common location of a spontaneous intracerebral hemorrhage (ICH) is the putamen, which is usually associated with uncontrolled hypertension.[9]
History and Physical
Epidural Hemorrhage
Patients with epidural hematoma report a history of a focal head injury, such as blunt trauma from a hammer or baseball bat, fall, or MVC. The classic presentation of an epidural hematoma is a loss of consciousness after the injury, followed by a lucid interval and then neurologic deterioration. This classic presentation occurs only in less than 20% of patients. Other common symptoms include severe headache, nausea, vomiting, lethargy, and seizure.
Subdural Hemorrhage
A history of either major or minor head injury can be found in cases of subdural hematoma. In older patients, a subdural hematoma can occur after trivial head injuries, including bumping of the head on a cabinet or running into a door or wall. An acute subdural can present with recent trauma, headache, nausea, vomiting, altered mental status, seizure, and/or lethargy. A chronic subdural hematoma can present with a headache, nausea, vomiting, confusion, decreased consciousness, lethargy, motor deficits, aphasia, seizure, or personality changes. A physical exam may demonstrate a focal motor deficit, neurologic deficits, lethargy, or altered consciousness.
Subarachnoid Hemorrhage
A thunderclap headache (sudden severe or worst headache of life) is the classic presentation of subarachnoid hemorrhage. Other symptoms include dizziness, nausea, vomiting, diplopia, seizures, loss of consciousness, or nuchal rigidity. Physical exam findings may include focal neurologic deficits, cranial nerve palsies, nuchal rigidity, or decreased or altered consciousness.
Intraparenchymal Hemorrhage
Non-traumatic intraparenchymal hemorrhages typically present with a history of sudden onset of stroke symptoms, including a headache, nausea, vomiting, focal neurologic deficits, lethargy, weakness, slurred speech, syncope, vertigo, or changes in sensation.
Evaluation
The initial evaluation, which encompasses a primary survey, prioritizes assessing the airway, breathing, and circulation. In addition, a comprehensive neurological examination is warranted to assess for focal neurological deficits.
Glasgow Coma Scale Score [10]
The Glasgow Coma Scale (GCS) helps quickly rate the severity of a patient's neurologic injury. The 3 components of this score are:
- Eye-opening (ranging from 1 to 4)
- Best verbal response (ranging from 1 to 5)
- Best motor response (ranging from 1 to 6)
TBI can be classified based on GCS as mild (13 to 15), moderate (9 to 12), and severe (8 or less).
Laboratory Studies
- Complete blood count to check for thrombocytopenia
- Coagulation studies (PTT, PT/INR) for coagulopathy
- A basic metabolic panel for electrolyte abnormalities
Imaging
A non-contrast computed tomography (CT) scan of the head is the imaging modality unless additional modalities are indicated for further evaluation.
Epidural Hemorrhage [11][12][13]
If intracranial pressure (ICP) increases, a Cushing response (hypertension, bradycardia, and bradypnea) may occur as compensation. The characteristic imaging finding is a hyperdense extra-axial lens-shaped (biconvex) opacity. Historically, cerebral angiography could locate the shift in cerebral blood vessels, but cerebral angiography is supplanted by CT imaging.
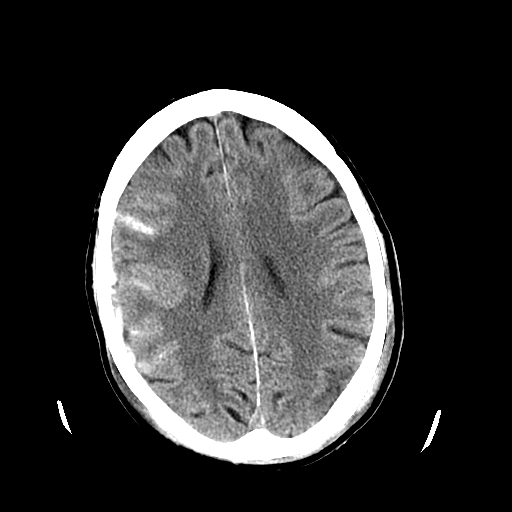
Subdural Hemorrhage
An acute subdural hematoma is typically hyperdense, while a chronic subdural is hypodense. A subacute subdural may be isodense to the brain and more challenging to identify (see Image. Subdural Hematoma Detected on Head CT).
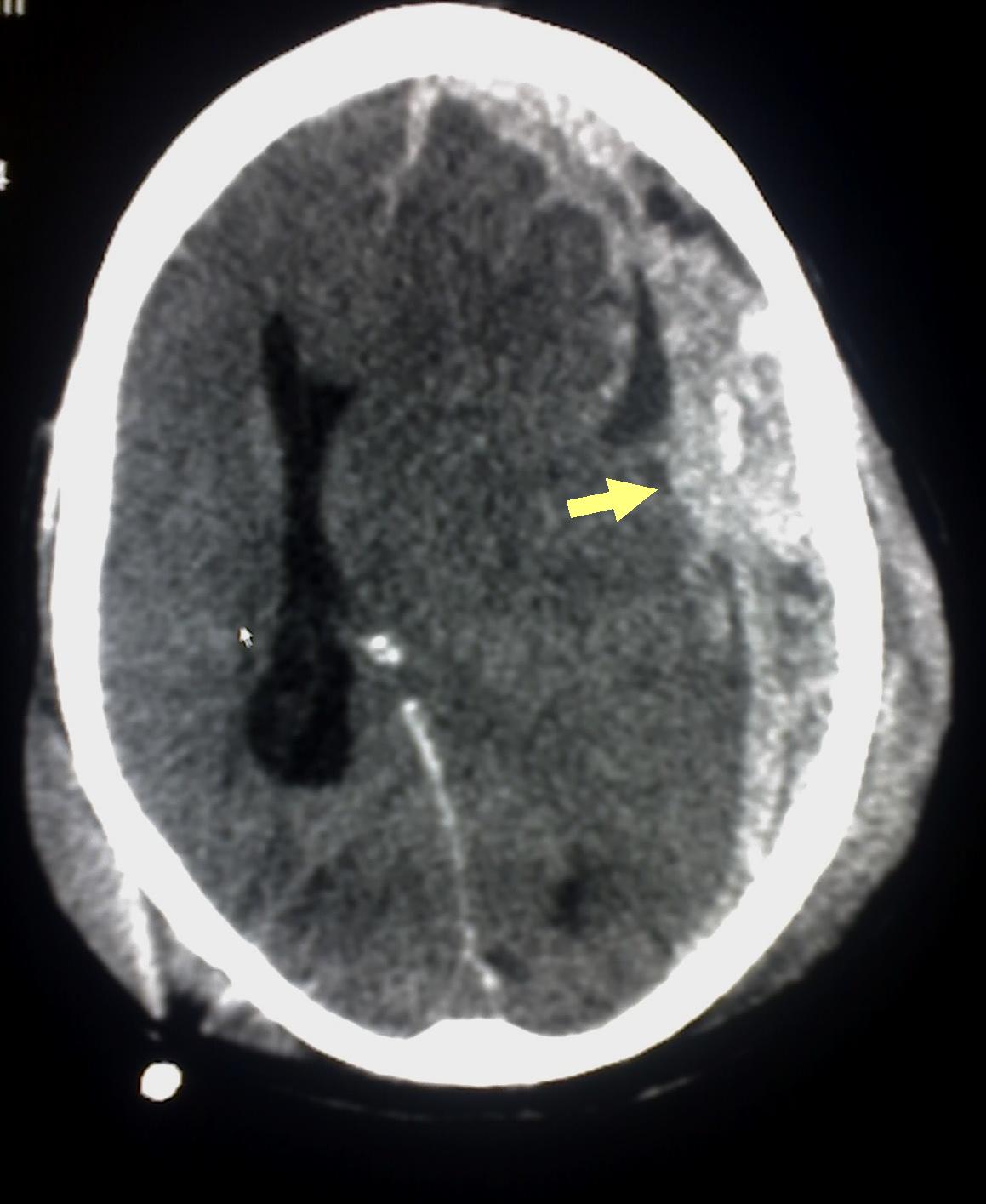
Subarachnoid Hemorrhage
Patients with subarachnoid hemorrhage can rapidly deteriorate and may need emergent intubation. If the patient is given contrast during imaging, this may obscure the subarachnoid hemorrhage. Acute subarachnoid hemorrhage is typically hyperdense on CT imaging (see Image. Head CT Revealing Subarachnoid Hemorrhage). If the CT scan is negative and a strong suspicion of subarachnoid hemorrhage exists, a lumbar puncture (LP) should be considered. The LP results may show xanthochromia. An LP performed before 6 hours of the subarachnoid hemorrhage may fail to show xanthochromia. Additionally, LP results may be confounded if a traumatic tap is encountered.
Identifying the cause of non-traumatic subarachnoid hemorrhage helps guide further treatment. Standard workup includes either a CT angiogram (CTA) of the head and neck, magnetic resonance angiography (MRA) of the head and neck, or diagnostic cerebral angiogram of the head and neck to look for an aneurysm, AVM, or another source of subarachnoid hemorrhage.
Intraparenchymal Hemorrhage
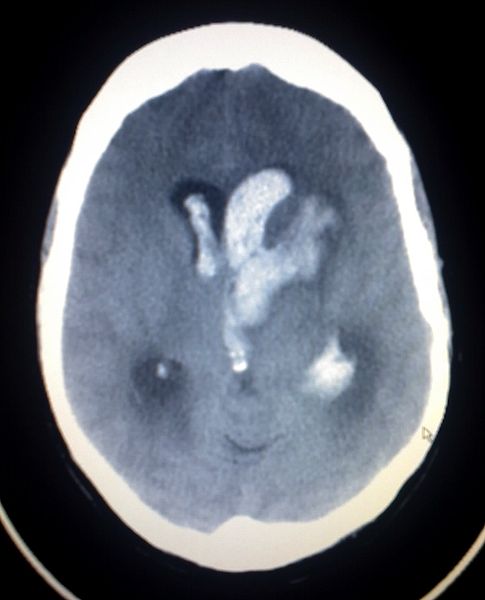
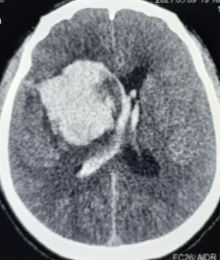
The imaging should be able to identify acute intraparenchymal hemorrhage as a hyperdensity within the parenchyma. Depending on the history and physical and imaging findings, a magnetic resonance imaging (MRI) scan of the brain with and without contrast should be considered. Head CT can also identify intraparenchymal hemorrhage (see Image. Intracerebral Hemorrhage with Intraventricular Bleeding) within specific regions (see Image. Intracranial Hemorrhage in the Caudate Region).
Other imaging to consider include CTA, MRA, or diagnostic cerebral angiogram to look for cerebrovascular causes of the intraparenchymal hemorrhage. Diagnostic cerebral angiography is indicated for all patients with ICH except those aged more than 45 with preexisting hypertension in thalamic, putaminal, or posterior fossa hemorrhage.[14] Cavernomas are angiographically occult and can be picked up incidentally or present with small parenchymal hemorrhage resulting in headaches, neurological deficits, or seizures. They have a characteristic "popcorn" or "berry" appearance on MRI scans with a rim of signal loss due to haemosiderin (see Image. Popcorn-Like Appearance of a Cavernoma).
Different intensities of blood products in different sequences of MRI scans[15]:
-
Hyperacute (<12 hours)
T1 Isointense
T2 hyperintense
Gradient echo - hypointense rim
-
Acute (12 hours to 2 days)
T1 Isointense
T2 hypointense
Gradient echo - a hypointense rim that gradually progresses to the center
-
Early subacute (2 to 7 days)
T1 hyperintense
T2 hypointense
Gradient echo - hypointense
-
Late subacute (8 days to 1 month)
T1 hyperintense
T2 hyperintense
Gradient echo - hyperintense with rim of low-intensity
-
Chronic (>1 month)
T1 hypointense
T2 hypointense
Gradient echo - slit-like hyperintense or isointense core surrounded by a hypointense rim
The most common type of intracranial hemorrhage in neonates is germinal matrix hemorrhage or periventricular-intraventricular hemorrhage (PVIH). PVIH is classified into the following grades (Papile):[16]
-
Grade I: Restricted to subependymal region/germinal matrix seen in the caudothalamic groove
-
Grade II: Extending into normal-sized ventricles and typically filling <50% of the ventricular volume
-
Grade III: Extensive hemorrhage with distension/dilation of the ventricles
-
Grade IV: Parenchymal involvement - has the highest mortality
Other
Asymptomatic striatocapsular slit-like hemorrhage has been studied as a severity marker in patients with hypertensive angiopathy (see Image. Chronic Hypertensive Hemorrhage).[17]
Remote cerebellar hemorrhage (RCH) typically occurs after supratentorial craniotomy and is less likely after spinal surgery. RCH occurs remotely near the surgical site and without an underlying pathologic lesion.[18]
Treatment / Management
Immediate neurosurgical consultation is recommended for all hemorrhages, but mainly if alarming signs such as airway compromise, respiratory failure, or hemodynamic instability exist.
Epidural Hemorrhage [19][20][21]
Definitive treatment is an evacuation of the hematoma and stopping the bleeding source. Some smaller epidural hematomas may be managed non-surgically and monitored for resolution.
Conservative management is attempted if the patient satisfies all of the following criteria and has mild symptoms[22]:
- Epidural hematoma volume of less than 30 mL
- A clot diameter of less than 15 mm
- Midline shift of less than 5 mm
- GCS greater than 8 and, on physical examination, shows no focal neurological symptoms.
Subdural Hemorrhage
Definitive treatment is an evacuation, but depending on the size and location, some subdural hematomas may be monitored for resolution. Non-surgical management options include repeat imaging to ensure subdural stability, anticoagulation reversal, platelet transfusions for thrombocytopenia or dysfunctional platelets, observation with frequent neurologic assessments for deterioration, and/or controlling hypertension. The use of steroids is still controversial.
Surgical management options include a twist drill hole, burr hole(s), and craniotomy for evacuation. Data suggests that a twist drill hole has the lowest surgical complication rate with the highest recurrence rate. A craniotomy has the highest surgical complication rate with the lowest recurrence rate of the surgical options, and burr hole(s) evacuation falls somewhere between a twist drill hole and a craniotomy for complication and recurrence rate.
Subarachnoid Hemorrhage
If the patient is on anticoagulation or antiplatelet agents, reversal is prioritized. Management is typically conservative with close assessments of vitals and neurologic status. In obtunded patients, a need for ICP monitoring and/or external ventricular drain (EVD) is common. Patients should be monitored for hydrocephalus or cerebral swelling. Repeat imaging can verify the improvement of the traumatic subarachnoid hemorrhage. Sometimes, aneurysmal rupture or incompetence of other intracranial vascular malformations can masquerade as traumatic subarachnoid hemorrhage. If no history of trauma is evident, then a non-traumatic etiology is considered.
In non-traumatic subarachnoid hemorrhage, the next steps vary depending on the etiology of the bleeding but can include treatment of an aneurysm, AVM, or other underlying pathology. Additionally, a low threshold for an EVD exists due to the risk of hydrocephalus.
Intraparenchymal Hemorrhage
Blood pressure should be monitored to decrease the risk of further hemorrhage. The treatment depends on the etiology of the hemorrhage. Treatment options are variable and include aggressive surgical evacuation, craniectomy, catheter-based dissolution, or observation. Surgical evacuation is controversial for some forms of intraparenchymal hemorrhage.
Although many intraparenchymal hemorrhages are secondary to cerebrovascular disease and hypertension, the surgeon should anticipate encountering underlying pathology, including an aneurysm, AVM, and/or tumor, when evacuating an intraparenchymal hemorrhage. Surgical evacuation is usually recommended for cerebellar hematomas larger than 3 cm in diameter[23], and an EVD is placed before the evacuation if hydrocephalus is evident.
Sometimes, evacuating the hematoma may be more detrimental than the hematoma itself, and a craniectomy is performed to promote cerebral swelling. Several catheter-based systems can dissolve the hemorrhage. Minor and non-operable hemorrhages may be non-surgically managed with blood pressure control, the reversal of anticoagulation or antiplatelet agents, and neuroprotective strategies to prevent and/or mitigate secondary cerebral injury.
The indications for surgical resection in cavernomas include:[24]
- Multiple hemorrhages in eloquent areas, or
- Single hemorrhage in a non-eloquent area associated with deteriorating neurological deficits
- Severe symptoms, such as cardiac or respiratory instability, and
- Cavernomas within 2 mm from the pial surface
Differential Diagnosis
The differential diagnoses of intracranial hemorrhage include:
- Infection: Subdural empyema may mimic a subdural hemorrhage
- Recent contrast administration
- Subdural hygroma: May appear similar to chronic subdural hemorrhage
- Tumors: Meningioma may mimic an extradural hemorrhage
Prognosis
The prognosis depends on several factors, including the age of the patient, comorbidities, consumption of antiplatelets or anticoagulants, Glasgow coma scale score at presentation, size of pupils at presentation, location of the bleed, presence of other injuries, and time delay for surgical intervention, if needed.
The ICH score is clinically significant for ICHs.
The ICH Score is the sum of individual points assigned as follows:[25]
- GCS score:
- 3 to 4=2 points
- 5 to 12=1 point
- 13 to 15=0 point
- Age 80 or older:
- Infratentorial origin:
- ICH volume:
- ≥30 cm3 =1 point
- <30 cm3 =0 point
- Intraventricular hemorrhage:
The mortality rate is calculated based on the ICH score as follows:
Complications
The main complications associated with intracranial hemorrhage include neurological deficits, brain herniation, infarcts, rebleeds, vasospasm, seizures, and death.
Deterrence and Patient Education
Patients should be educated on the importance of rehabilitation and medication compliance during recovery. They cannot drive unless cleared.
Enhancing Healthcare Team Outcomes
Patients presenting with bleeding in the central nervous system are optimally treated by an interprofessional healthcare team comprising a neurologist, neurosurgeon, radiologist, intensivist, neurosurgery nurses, physical therapist, pulmonologist, and various allied health specialists such as speech, occupational, and physical therapists. Admission to the intensive care unit (ICU) is often necessary for many of these patients, and their care is tailored based on the severity of physical and neurological deficits. Factors including the type and extent of the bleed, patient age, comorbidities, and the severity of neurological deficits upon admission influence prognosis.[26][7][27]