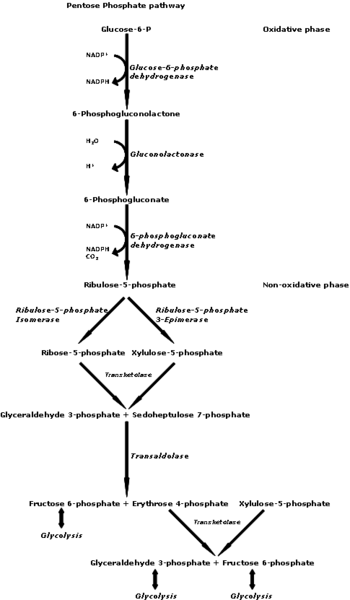
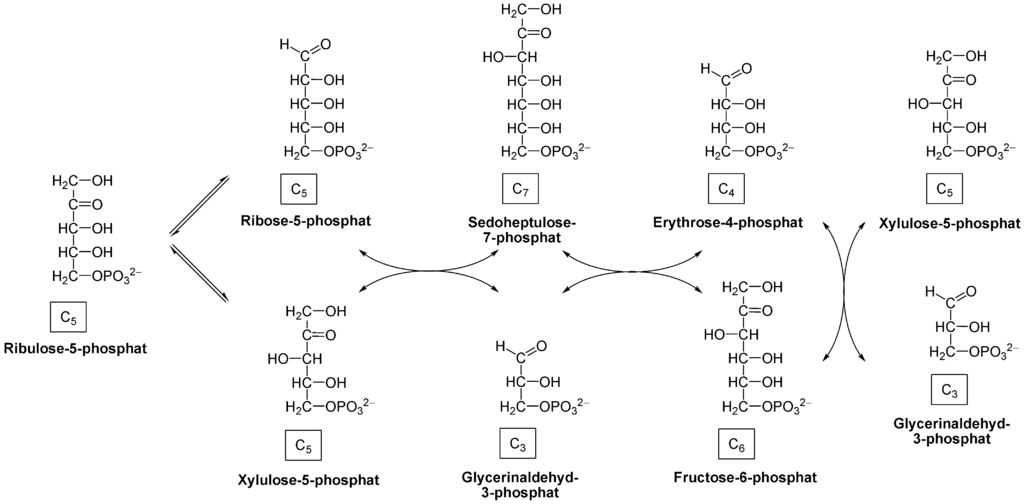
[1]
DE LOECKER WC, PRANKERD TA. Factors influencing the hexose monophosphate shunt in red cells. Clinica chimica acta; international journal of clinical chemistry. 1961 Sep:6():641-7
[PubMed PMID: 13884322]
[2]
ROSSI F, ZATTI M, GREENBAUM AL. Evidence for the existence of the hexose monophosphate pathway for glucose metabolism in the normal and denervated skeletal muscle of rats. The Biochemical journal. 1963 Apr:87(1):43-8
[PubMed PMID: 13975179]
[3]
Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biological reviews of the Cambridge Philosophical Society. 2015 Aug:90(3):927-63. doi: 10.1111/brv.12140. Epub 2014 Sep 22
[PubMed PMID: 25243985]
[4]
Tsan MF, McIntyre PA. Stimulation by propylthiouracil of the hexose monophosphate shunt in human polymorphonuclear leucocytes during phagocytosis. British journal of haematology. 1975 Oct:31(2):193-208
[PubMed PMID: 1201238]
[5]
Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends in molecular medicine. 2011 Jul:17(7):395-403. doi: 10.1016/j.molmed.2011.01.014. Epub 2011 Mar 2
[PubMed PMID: 21376665]
[6]
Guitton J, Servanin S, Francina A. Hexose monophosphate shunt activities in human erythrocytes during oxidative damage induced by hydrogen peroxide. Archives of toxicology. 2003 Jul:77(7):410-7
[PubMed PMID: 12851742]
[7]
Jacobasch G, Bleiber R, Schönian G. Metabolism of the hexose monophosphate shunt in glucose-6-phosphate dehydrogenase deficiency and closely interrelated reactions. Haematologia. 1982 Dec:15(4):401-7
[PubMed PMID: 7186479]
[9]
Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB life. 2012 May:64(5):362-9. doi: 10.1002/iub.1017. Epub 2012 Mar 20
[PubMed PMID: 22431005]
[11]
Novello F, McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. The Biochemical journal. 1968 May:107(6):775-91
[PubMed PMID: 16742603]