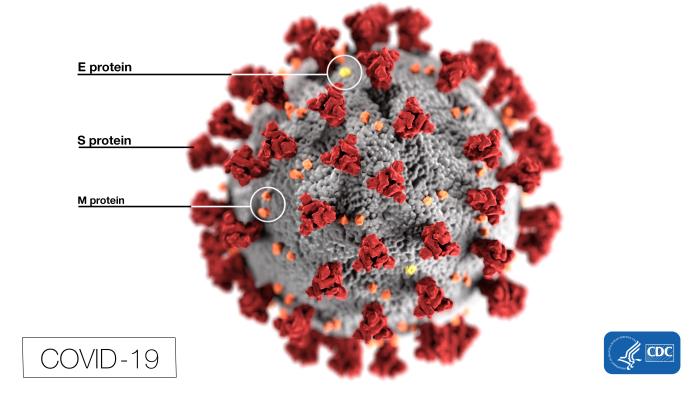
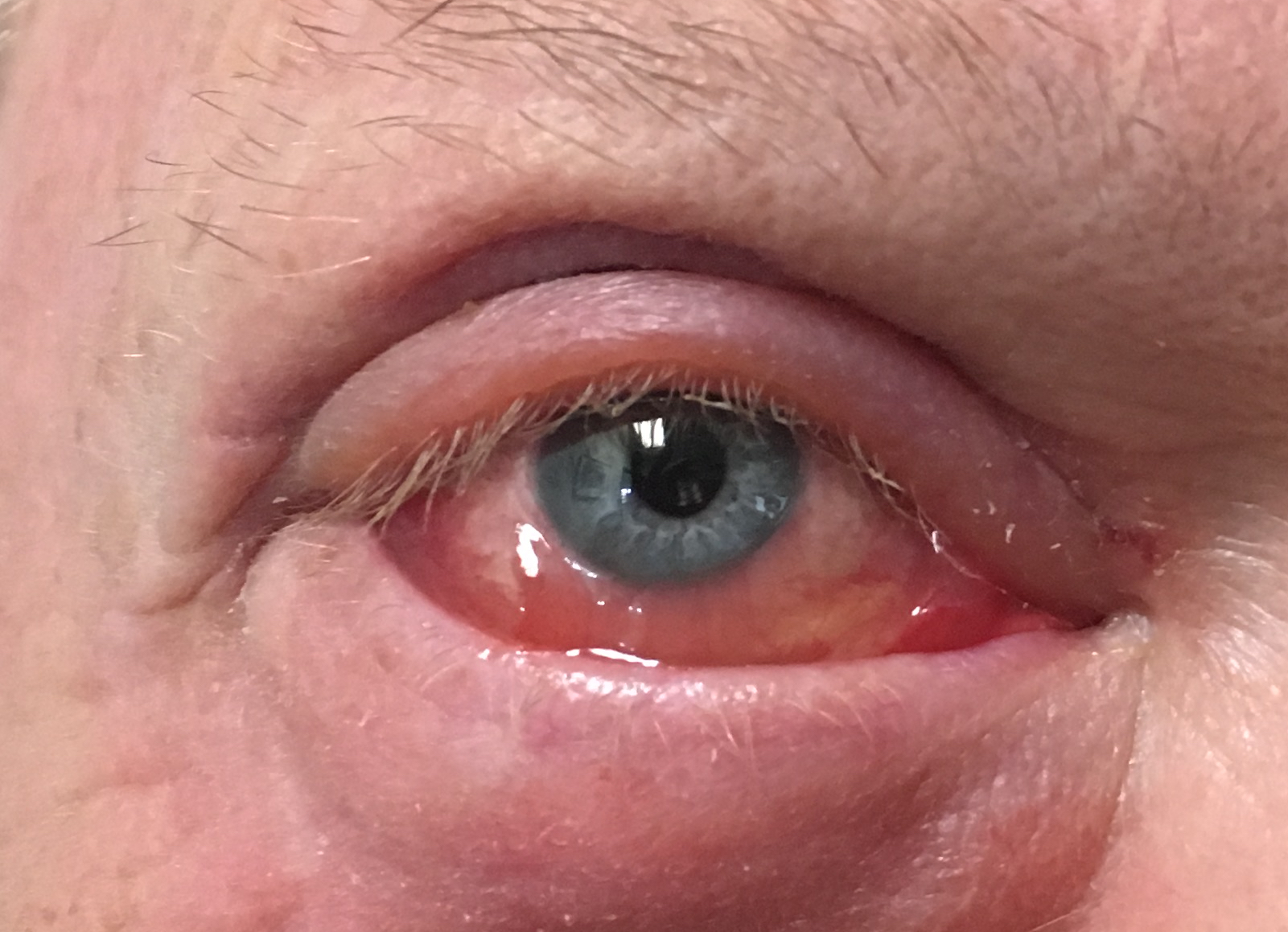
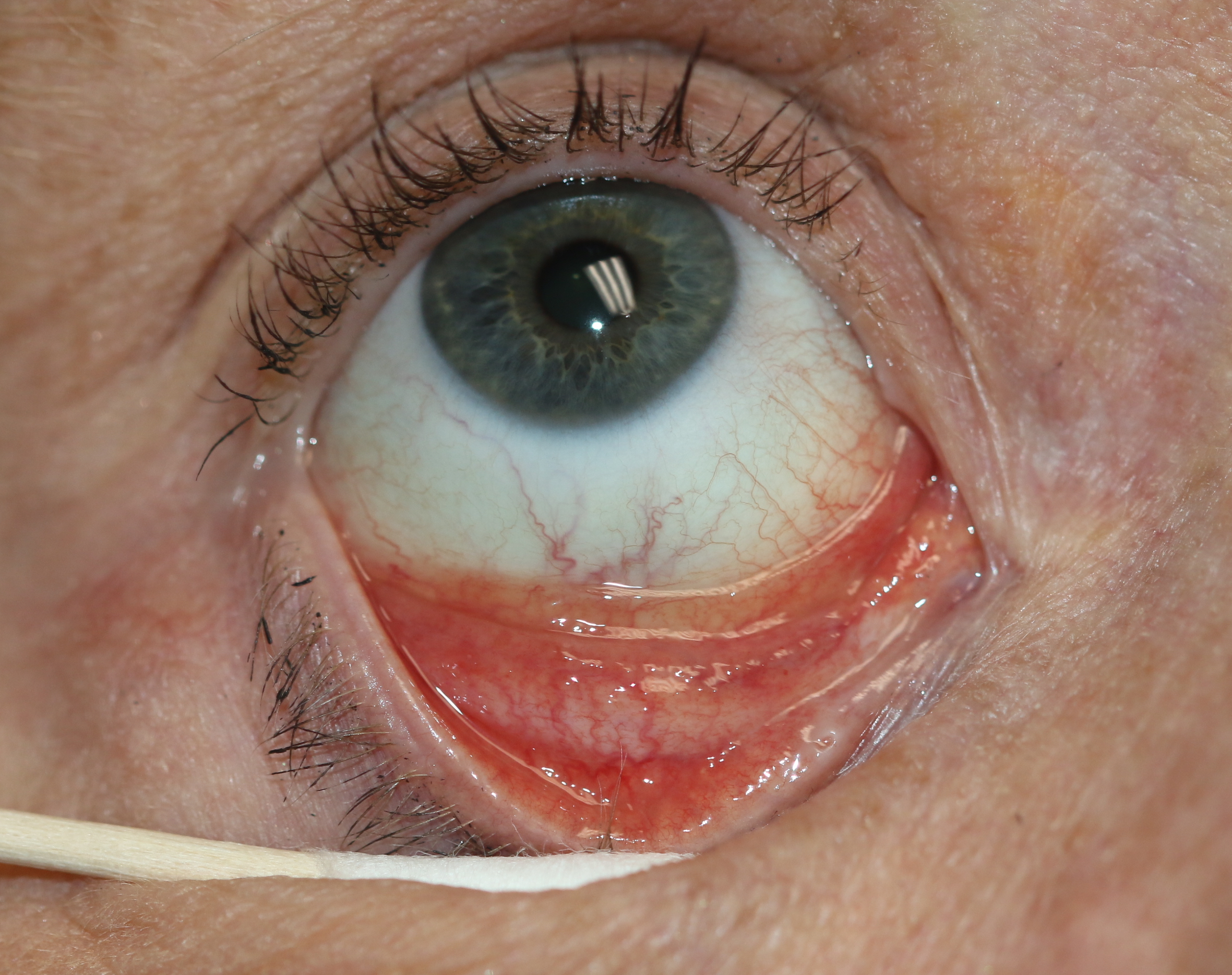
[1]
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls. 2024 Jan:():
[PubMed PMID: 32150360]
[2]
Loon SC, Teoh SC, Oon LL, Se-Thoe SY, Ling AE, Leo YS, Leong HN. The severe acute respiratory syndrome coronavirus in tears. The British journal of ophthalmology. 2004 Jul:88(7):861-3
[PubMed PMID: 15205225]
[3]
Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, Gravel D, Henry B, Lapinsky S, Loeb M, McDonald LC, Ofner M, Paton S, Reynolds D, Scales D, Shen S, Simor A, Stewart T, Vearncombe M, Zoutman D, Green K. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS one. 2010 May 19:5(5):e10717. doi: 10.1371/journal.pone.0010717. Epub 2010 May 19
[PubMed PMID: 20502660]
[4]
Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet (London, England). 2020 Feb 22:395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. Epub 2020 Feb 6
[PubMed PMID: 32035510]
[5]
Azzolini C, Donati S, Premi E, Baj A, Siracusa C, Genoni A, Grossi PA, Azzi L, Sessa F, Dentali F, Severgnini P, Minoja G, Cabrini L, Chiaravalli M, Veronesi G, Carcano G, Maffioli LS, Tagliabue A. SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients With COVID-19 From the Lombardy Region, Italy. JAMA ophthalmology. 2021 Sep 1:139(9):956-963. doi: 10.1001/jamaophthalmol.2020.5464. Epub
[PubMed PMID: 33662099]
[6]
Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocular immunology and inflammation. 2020 Apr 2:28(3):391-395. doi: 10.1080/09273948.2020.1738501. Epub 2020 Mar 16
[PubMed PMID: 32175797]
Level 3 (low-level) evidence
[7]
Li JO, Lam DSC, Chen Y, Ting DSW. Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear. The British journal of ophthalmology. 2020 Mar:104(3):297-298. doi: 10.1136/bjophthalmol-2020-315994. Epub
[PubMed PMID: 32086236]
[8]
Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clinics and practice. 2021 Oct 21:11(4):778-784. doi: 10.3390/clinpract11040093. Epub 2021 Oct 21
[PubMed PMID: 34698149]
[9]
Ferré VM, Peiffer-Smadja N, Visseaux B, Descamps D, Ghosn J, Charpentier C. Omicron SARS-CoV-2 variant: What we know and what we don't. Anaesthesia, critical care & pain medicine. 2022 Feb:41(1):100998. doi: 10.1016/j.accpm.2021.100998. Epub 2021 Dec 10
[PubMed PMID: 34902630]
[10]
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020 Apr 30:382(18):1708-1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28
[PubMed PMID: 32109013]
Level 2 (mid-level) evidence
[11]
Nasiri N, Sharifi H, Bazrafshan A, Noori A, Karamouzian M, Sharifi A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-analysis. Journal of ophthalmic & vision research. 2021 Jan-Mar:16(1):103-112. doi: 10.18502/jovr.v16i1.8256. Epub 2021 Jan 20
[PubMed PMID: 33520133]
Level 1 (high-level) evidence
[12]
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA ophthalmology. 2020 May 1:138(5):575-578. doi: 10.1001/jamaophthalmol.2020.1291. Epub
[PubMed PMID: 32232433]
[13]
Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. Journal of medical virology. 2020 Jun:92(6):589-594. doi: 10.1002/jmv.25725. Epub 2020 Mar 12
[PubMed PMID: 32100876]
[14]
Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends in immunology. 2020 Dec:41(12):1100-1115. doi: 10.1016/j.it.2020.10.004. Epub 2020 Oct 14
[PubMed PMID: 33132005]
[15]
Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. bioRxiv : the preprint server for biology. 2020 May 9:():. pii: 2020.05.09.086165. doi: 10.1101/2020.05.09.086165. Epub 2020 May 9
[PubMed PMID: 32511393]
[16]
Lange C, Wolf J, Auw-Haedrich C, Schlecht A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T, Schlunck G. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. Journal of medical virology. 2020 Oct:92(10):2081-2086. doi: 10.1002/jmv.25981. Epub 2020 Jul 11
[PubMed PMID: 32374427]
[17]
Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, Nicastri E, Bevilacqua N, Giancola ML, Corpolongo A, Ippolito G, Capobianchi MR, Castilletti C. SARS-CoV-2 Isolation From Ocular Secretions of a Patient With COVID-19 in Italy With Prolonged Viral RNA Detection. Annals of internal medicine. 2020 Aug 4:173(3):242-243. doi: 10.7326/M20-1176. Epub 2020 Apr 17
[PubMed PMID: 32302380]
[18]
Chen MJ, Chang KJ, Hsu CC, Lin PY, Jui-Ling Liu C. Precaution and prevention of coronavirus disease 2019 infection in the eye. Journal of the Chinese Medical Association : JCMA. 2020 Jul:83(7):648-650. doi: 10.1097/JCMA.0000000000000334. Epub
[PubMed PMID: 32332516]
[19]
Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The Ocular Manifestations and Transmission of COVID-19: Recommendations for Prevention. The Journal of emergency medicine. 2020 Jul:59(1):137-140. doi: 10.1016/j.jemermed.2020.04.060. Epub 2020 May 8
[PubMed PMID: 32456959]
[20]
Jevnikar K, Jaki Mekjavic P, Vidovic Valentincic N, Petrovski G, Globocnik Petrovic M. An Update on COVID-19 Related Ophthalmic Manifestations. Ocular immunology and inflammation. 2021 May 19:29(4):684-689. doi: 10.1080/09273948.2021.1896008. Epub 2021 Apr 7
[PubMed PMID: 33826465]
[21]
Kumar KK, Sampritha UC, Prakash AA, Adappa K, Chandraprabha S, Neeraja TG, Guru Prasad NS, Basumatary J, Gangasagara SB, Sujatha Rathod BL, Jayanthi CR. Ophthalmic manifestations in the COVID-19 clinical spectrum. Indian journal of ophthalmology. 2021 Mar:69(3):691-694. doi: 10.4103/ijo.IJO_3037_20. Epub
[PubMed PMID: 33595502]
[22]
Soltani S, Tabibzadeh A, Zakeri A, Zakeri AM, Latifi T, Shabani M, Pouremamali A, Erfani Y, Pakzad I, Malekifar P, Valizadeh R, Zandi M, Pakzad R. COVID-19 associated central nervous system manifestations, mental and neurological symptoms: a systematic review and meta-analysis. Reviews in the neurosciences. 2021 Apr 27:32(3):351-361. doi: 10.1515/revneuro-2020-0108. Epub 2021 Jan 13
[PubMed PMID: 33618441]
Level 1 (high-level) evidence
[23]
Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian journal of ophthalmology. 2021 Mar:69(3):488-509. doi: 10.4103/ijo.IJO_297_21. Epub
[PubMed PMID: 33595463]
[24]
Domínguez-Varela IA, Rodríguez-Gutiérrez LA, Morales-Mancillas NR, Barrera-Sánchez M, Macías-Rodríguez Y, Valdez-García JE. COVID-19 and the eye: a review. Infectious diseases (London, England). 2021 Jun:53(6):399-403. doi: 10.1080/23744235.2021.1882697. Epub 2021 Feb 10
[PubMed PMID: 33566704]
[25]
Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020 Jun:258(6):1351-1352. doi: 10.1007/s00417-020-04695-8. Epub 2020 Apr 23
[PubMed PMID: 32328758]
[26]
Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020:20():e00774. doi: 10.1016/j.idcr.2020.e00774. Epub 2020 Apr 24
[PubMed PMID: 32373467]
Level 3 (low-level) evidence
[27]
Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2020 Aug:55(4):e125-e129. doi: 10.1016/j.jcjo.2020.03.003. Epub 2020 Apr 2
[PubMed PMID: 32284146]
[28]
Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. American journal of ophthalmology case reports. 2020 Sep:19():100735. doi: 10.1016/j.ajoc.2020.100735. Epub 2020 May 6
[PubMed PMID: 32377594]
Level 3 (low-level) evidence
[29]
Danthuluri V, Grant MB. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmology and therapy. 2020 Dec:9(4):853-875. doi: 10.1007/s40123-020-00310-5. Epub 2020 Oct 15
[PubMed PMID: 33058068]
Level 3 (low-level) evidence
[30]
Otaif W, Al Somali AI, Al Habash A. Episcleritis as a possible presenting sign of the novel coronavirus disease: A case report. American journal of ophthalmology case reports. 2020 Dec:20():100917. doi: 10.1016/j.ajoc.2020.100917. Epub 2020 Sep 8
[PubMed PMID: 32923742]
Level 3 (low-level) evidence
[31]
Méndez Mangana C, Barraquer Kargacin A, Barraquer RI. Episcleritis as an ocular manifestation in a patient with COVID-19. Acta ophthalmologica. 2020 Dec:98(8):e1056-e1057. doi: 10.1111/aos.14484. Epub 2020 Jun 1
[PubMed PMID: 32483943]
[32]
Feizi S, Meshksar A, Naderi A, Esfandiari H. Anterior Scleritis Manifesting After Coronavirus Disease 2019: A Report of Two Cases. Cornea. 2021 Sep 1:40(9):1204-1206. doi: 10.1097/ICO.0000000000002795. Epub
[PubMed PMID: 34351874]
Level 3 (low-level) evidence
[33]
Mazzotta C, Giancipoli E. Anterior Acute Uveitis Report in a SARS-CoV-2 Patient Managed with Adjunctive Topical Antiseptic Prophylaxis Preventing 2019-nCoV Spread Through the Ocular Surface Route. International medical case reports journal. 2020:13():513-520. doi: 10.2147/IMCRJ.S260252. Epub 2020 Oct 13
[PubMed PMID: 33116943]
Level 3 (low-level) evidence
[34]
Bettach E, Zadok D, Weill Y, Brosh K, Hanhart J. Bilateral anterior uveitis as a part of a multisystem inflammatory syndrome secondary to COVID-19 infection. Journal of medical virology. 2021 Jan:93(1):139-140. doi: 10.1002/jmv.26229. Epub 2020 Sep 30
[PubMed PMID: 32592496]
[35]
Sanjay S, Mutalik D, Gowda S, Mahendradas P, Kawali A, Shetty R. "Post Coronavirus Disease (COVID-19) Reactivation of a Quiescent Unilateral Anterior Uveitis". SN comprehensive clinical medicine. 2021:3(9):1843-1847. doi: 10.1007/s42399-021-00985-2. Epub 2021 Jun 7
[PubMed PMID: 34124585]
[36]
Zhou L, Xu Z, Guerra J, Rosenberg AZ, Fenaroli P, Eberhart CG, Duh EJ. Expression of the SARS-CoV-2 Receptor ACE2 in Human Retina and Diabetes-Implications for Retinopathy. Investigative ophthalmology & visual science. 2021 Jun 1:62(7):6. doi: 10.1167/iovs.62.7.6. Epub
[PubMed PMID: 34086044]
[37]
Araujo-Silva CA, Marcos AAA, Marinho PM, Branco AMC, Roque A, Romano AC, Matuoka ML, Farah M, Burnier M, Moraes NF, Tierno PFGMM, Schor P, Sakamoto V, Nascimento H, de Sousa W, Belfort R Jr. Presumed SARS-CoV-2 Viral Particles in the Human Retina of Patients With COVID-19. JAMA ophthalmology. 2021 Sep 1:139(9):1015-1021. doi: 10.1001/jamaophthalmol.2021.2795. Epub
[PubMed PMID: 34323931]
[38]
Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian journal of ophthalmology. 2020 Nov:68(11):2572-2574. doi: 10.4103/ijo.IJO_2575_20. Epub
[PubMed PMID: 33120696]
[39]
Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: A case report. American journal of ophthalmology case reports. 2020 Dec:20():100992. doi: 10.1016/j.ajoc.2020.100992. Epub 2020 Nov 16
[PubMed PMID: 33225111]
Level 3 (low-level) evidence
[40]
Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020:21():e00867. doi: 10.1016/j.idcr.2020.e00867. Epub 2020 Jun 18
[PubMed PMID: 32572363]
Level 3 (low-level) evidence
[41]
Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case Report: Central Retinal Artery Occlusion in a COVID-19 Patient. Frontiers in pharmacology. 2020:11():588384. doi: 10.3389/fphar.2020.588384. Epub 2020 Dec 23
[PubMed PMID: 33424598]
Level 3 (low-level) evidence
[42]
Murchison AP, Sweid A, Dharia R, Theofanis TN, Tjoumakaris SI, Jabbour PM, Bilyk JR. Monocular visual loss as the presenting symptom of COVID-19 infection. Clinical neurology and neurosurgery. 2021 Feb:201():106440. doi: 10.1016/j.clineuro.2020.106440. Epub 2020 Dec 15
[PubMed PMID: 33383464]
[43]
Gascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M, Sauvan L, Swiader L, Durand JM, Denis D. Covid-19-Associated Retinopathy: A Case Report. Ocular immunology and inflammation. 2020 Nov 16:28(8):1293-1297. doi: 10.1080/09273948.2020.1825751. Epub 2020 Oct 6
[PubMed PMID: 33021856]
Level 3 (low-level) evidence
[44]
Bottini AR, Steinmetz S, Blinder KJ, Shah GK. Purtscher-Like Retinopathy in a Patient with COVID-19. Case reports in ophthalmological medicine. 2021:2021():6661541. doi: 10.1155/2021/6661541. Epub 2021 Mar 20
[PubMed PMID: 33859855]
Level 3 (low-level) evidence
[45]
Rahman EZ, Shah P, Ong JE, Goldberg M, Ong SS. Purtscher-like retinopathy in a patient with COVID-19 and disseminated intravascular coagulation. American journal of ophthalmology case reports. 2021 Dec:24():101229. doi: 10.1016/j.ajoc.2021.101229. Epub 2021 Nov 11
[PubMed PMID: 34796309]
Level 3 (low-level) evidence
[46]
Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R Jr. Retinal findings in patients with COVID-19. Lancet (London, England). 2020 May 23:395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. Epub 2020 May 12
[PubMed PMID: 32405105]
[47]
Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V, Giacomelli A, Cigada M, Milazzo L, Ridolfo A, Faggion I, Cordier L, Oldani M, Marini S, Villa P, Rizzardini G, Galli M, Antinori S, Staurenghi G, Meroni L. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020 Oct:27():100550. doi: 10.1016/j.eclinm.2020.100550. Epub 2020 Sep 20
[PubMed PMID: 32984785]
[48]
Lecler A, Cotton F, Lersy F, Kremer S, Héran F, SFNR's COVID Study Group. Ocular MRI Findings in Patients with Severe COVID-19: A Retrospective Multicenter Observational Study. Radiology. 2021 May:299(2):E226-E229. doi: 10.1148/radiol.2021204394. Epub 2021 Feb 16
[PubMed PMID: 33591889]
Level 2 (mid-level) evidence
[49]
de Souza EC, de Campos VE, Duker JS. Atypical unilateral multifocal choroiditis in a COVID-19 positive patient. American journal of ophthalmology case reports. 2021 Jun:22():101034. doi: 10.1016/j.ajoc.2021.101034. Epub 2021 Feb 19
[PubMed PMID: 33623832]
Level 3 (low-level) evidence
[50]
Goyal M, Murthy SI, Annum S. Bilateral Multifocal Choroiditis following COVID-19 Vaccination. Ocular immunology and inflammation. 2021 May 19:29(4):753-757. doi: 10.1080/09273948.2021.1957123. Epub 2021 Aug 3
[PubMed PMID: 34344280]
[51]
Providência J, Fonseca C, Henriques F, Proença R. Serpiginous choroiditis presenting after SARS-CoV-2 infection: A new immunological trigger? European journal of ophthalmology. 2022 Jan:32(1):NP97-NP101. doi: 10.1177/1120672120977817. Epub 2020 Dec 2
[PubMed PMID: 33267645]
[52]
Tom ES, McKay KM, Saraf SS. Bilateral Ampiginous Choroiditis following Presumed SARS-CoV-2 Infection. Case reports in ophthalmological medicine. 2021:2021():1646364. doi: 10.1155/2021/1646364. Epub 2021 Aug 5
[PubMed PMID: 34367705]
Level 3 (low-level) evidence
[53]
Soni A, Narayanan R, Tyagi M, Belenje A, Basu S. Acute Retinal Necrosis as a presenting ophthalmic manifestation in COVID 19 recovered patients. Ocular immunology and inflammation. 2021 May 19:29(4):722-725. doi: 10.1080/09273948.2021.1938135. Epub 2021 Jul 6
[PubMed PMID: 34228583]
[54]
Chin MS, Hooper LC, Hooks JJ, Detrick B. Identification of α-fodrin as an autoantigen in experimental coronavirus retinopathy (ECOR). Journal of neuroimmunology. 2014 Jul 15:272(1-2):42-50. doi: 10.1016/j.jneuroim.2014.05.002. Epub 2014 May 10
[PubMed PMID: 24864013]
[55]
Wang Y, Detrick B, Yu ZX, Zhang J, Chesky L, Hooks JJ. The role of apoptosis within the retina of coronavirus-infected mice. Investigative ophthalmology & visual science. 2000 Sep:41(10):3011-8
[PubMed PMID: 10967058]
[56]
Vinores SA, Wang Y, Vinores MA, Derevjanik NL, Shi A, Klein DA, Detrick B, Hooks JJ. Blood-retinal barrier breakdown in experimental coronavirus retinopathy: association with viral antigen, inflammation, and VEGF in sensitive and resistant strains. Journal of neuroimmunology. 2001 Oct 1:119(2):175-82
[PubMed PMID: 11585619]
[57]
Luís ME, Hipólito-Fernandes D, Mota C, Maleita D, Xavier C, Maio T, Cunha JP, Tavares Ferreira J. A Review of Neuro-Ophthalmological Manifestations of Human Coronavirus Infection. Eye and brain. 2020:12():129-137. doi: 10.2147/EB.S268828. Epub 2020 Oct 30
[PubMed PMID: 33154692]
[58]
Sawalha K, Adeodokun S, Kamoga GR. COVID-19-Induced Acute Bilateral Optic Neuritis. Journal of investigative medicine high impact case reports. 2020 Jan-Dec:8():2324709620976018. doi: 10.1177/2324709620976018. Epub
[PubMed PMID: 33238757]
Level 3 (low-level) evidence
[59]
Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020 Sep:40(3):398-402. doi: 10.1097/WNO.0000000000001049. Epub
[PubMed PMID: 32604245]
[60]
de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Multiple sclerosis and related disorders. 2020 Nov:46():102474. doi: 10.1016/j.msard.2020.102474. Epub 2020 Sep 1
[PubMed PMID: 32892062]
Level 3 (low-level) evidence
[61]
Leber HM, Sant'Ana L, Konichi da Silva NR, Raio MC, Mazzeo TJMM, Endo CM, Nascimento H, de Souza CE. Acute Thyroiditis and Bilateral Optic Neuritis following SARS-CoV-2 Vaccination with CoronaVac: A Case Report. Ocular immunology and inflammation. 2021 Aug 18:29(6):1200-1206. doi: 10.1080/09273948.2021.1961815. Epub 2021 Aug 17
[PubMed PMID: 34402726]
Level 3 (low-level) evidence
[62]
Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Multiple sclerosis and related disorders. 2020 Oct:45():102377. doi: 10.1016/j.msard.2020.102377. Epub 2020 Jul 7
[PubMed PMID: 32698095]
[63]
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA, Radmanesh A, Nelson PK. Cerebral Venous Thrombosis Associated with COVID-19. AJNR. American journal of neuroradiology. 2020 Aug:41(8):1370-1376. doi: 10.3174/ajnr.A6644. Epub 2020 Jun 18
[PubMed PMID: 32554424]
[64]
Baccarella A, Linder A, Spencer R, Jonokuchi AJ, King PB, Maldonado-Soto A, Boneparth A, Hooe BS, Schweickert AJ, Carlin RF, Kingery F, Vargas WS, Sewell TB, Silver WG. Increased Intracranial Pressure in the Setting of Multisystem Inflammatory Syndrome in Children, Associated With COVID-19. Pediatric neurology. 2021 Feb:115():48-49. doi: 10.1016/j.pediatrneurol.2020.11.008. Epub 2020 Nov 22
[PubMed PMID: 33333460]
[65]
Verkuil LD, Liu GT, Brahma VL, Avery RA. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet (London, England). 2020 Aug 22:396(10250):532. doi: 10.1016/S0140-6736(20)31725-6. Epub 2020 Aug 11
[PubMed PMID: 32795406]
Level 3 (low-level) evidence
[66]
Belghmaidi S, Nassih H, Boutgayout S, El Fakiri K, El Qadiry R, Hajji I, Bourrahouate A, Moutaouakil A. Third Cranial Nerve Palsy Presenting with Unilateral Diplopia and Strabismus in a 24-Year-Old Woman with COVID-19. The American journal of case reports. 2020 Oct 15:21():e925897. doi: 10.12659/AJCR.925897. Epub 2020 Oct 15
[PubMed PMID: 33056942]
Level 3 (low-level) evidence
[67]
Oliveira RMC, Santos DH, Olivetti BC, Takahashi JT. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arquivos de neuro-psiquiatria. 2020 Jun:78(6):385-386. doi: 10.1590/0004-282X20200052. Epub
[PubMed PMID: 32609196]
[68]
Greer CE, Bhatt JM, Oliveira CA, Dinkin MJ. Isolated Cranial Nerve 6 Palsy in 6 Patients With COVID-19 Infection. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020 Dec:40(4):520-522. doi: 10.1097/WNO.0000000000001146. Epub
[PubMed PMID: 32941331]
[69]
Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, Harpe J, Greer C, Mints G, Salama G, Tsiouris AJ, Leifer D. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 Aug 4:95(5):221-223. doi: 10.1212/WNL.0000000000009700. Epub 2020 May 1
[PubMed PMID: 32358218]
[70]
Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia Gravis Associated With SARS-CoV-2 Infection. Annals of internal medicine. 2020 Dec 15:173(12):1027-1028. doi: 10.7326/L20-0845. Epub 2020 Aug 10
[PubMed PMID: 32776781]
[71]
Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K, Wegner F. Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Frontiers in neurology. 2020:11():576153. doi: 10.3389/fneur.2020.576153. Epub 2020 Oct 6
[PubMed PMID: 33123081]
[72]
Ortiz-Seller A, Martínez Costa L, Hernández-Pons A, Valls Pascual E, Solves Alemany A, Albert-Fort M. Ophthalmic and Neuro-ophthalmic Manifestations of Coronavirus Disease 2019 (COVID-19). Ocular immunology and inflammation. 2020 Nov 16:28(8):1285-1289. doi: 10.1080/09273948.2020.1817497. Epub 2020 Oct 6
[PubMed PMID: 33021422]
[73]
Ordás CM, Villacieros-Álvarez J, Pastor-Vivas AI, Corrales-Benítez Á. Concurrent tonic pupil and trochlear nerve palsy in COVID-19. Journal of neurovirology. 2020 Dec:26(6):970-972. doi: 10.1007/s13365-020-00909-1. Epub 2020 Sep 10
[PubMed PMID: 32910433]
[74]
Kaya Tutar N, Kale N, Tugcu B. Adie-Holmes syndrome associated with COVID-19 infection: A case report. Indian journal of ophthalmology. 2021 Mar:69(3):773-774. doi: 10.4103/ijo.IJO_3589_20. Epub
[PubMed PMID: 33595525]
Level 3 (low-level) evidence
[75]
Malayala SV, Raza A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus. 2020 Jun 30:12(6):e8918. doi: 10.7759/cureus.8918. Epub 2020 Jun 30
[PubMed PMID: 32760619]
Level 3 (low-level) evidence
[76]
Wong PF, Craik S, Newman P, Makan A, Srinivasan K, Crawford E, Dev D, Moudgil H, Ahmad N. Lessons of the month 1: A case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clinical medicine (London, England). 2020 May 15:20(3):293-294. doi: 10.7861/clinmed.2020-0182. Epub 2020 May 15
[PubMed PMID: 32371417]
Level 3 (low-level) evidence
[77]
Llorente Ayuso L, Torres Rubio P, Beijinho do Rosário RF, Giganto Arroyo ML, Sierra-Hidalgo F. Bickerstaff encephalitis after COVID-19. Journal of neurology. 2021 Jun:268(6):2035-2037. doi: 10.1007/s00415-020-10201-1. Epub 2020 Sep 3
[PubMed PMID: 32880723]
[78]
Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, Kahan J, Lansdale KN, LeMoss NM, Murthy SB, Stieg PE, Fink ME, Iadecola C, Segal AZ, Cusick M, Campion TR Jr, Diaz I, Zhang C, Navi BB. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA neurology. 2020 Jul 2:77(11):1-7. doi: 10.1001/jamaneurol.2020.2730. Epub 2020 Jul 2
[PubMed PMID: 32614385]
[79]
Wilkinson SW, Etheridge T, Swiston CJ, Vegunta S, Wiggins RH, Warner JEA. Bilateral Posterior Cerebral Artery Stroke from COVID-Related Multisystem Inflammatory Syndrome in a Child. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2022 Sep 1:42(3):e548-e550. doi: 10.1097/WNO.0000000000001468. Epub 2021 Nov 11
[PubMed PMID: 34812758]
[80]
Turbin RE, Wawrzusin PJ, Sakla NM, Traba CM, Wong KG, Mirani N, Eloy JA, Nimchinsky EA. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit (Amsterdam, Netherlands). 2020 Aug:39(4):305-310. doi: 10.1080/01676830.2020.1768560. Epub 2020 May 18
[PubMed PMID: 32419568]
[81]
Shires CB, Klug T, Dryden S, Ford J. Unusual cause of acute sinusitis and orbital abscess in COVID-19 positive patient: Case report. International journal of surgery case reports. 2021 Feb:79():164-168. doi: 10.1016/j.ijscr.2021.01.043. Epub 2021 Jan 15
[PubMed PMID: 33477076]
Level 3 (low-level) evidence
[82]
Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, Simko JP, Winn BJ. Acute Invasive Rhino-Orbital Mucormycosis in a Patient With COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic plastic and reconstructive surgery. 2021 Mar-Apr 01:37(2):e40-e80. doi: 10.1097/IOP.0000000000001889. Epub
[PubMed PMID: 33229953]
[83]
Mehta S, Pandey A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus. 2020 Sep 30:12(9):e10726. doi: 10.7759/cureus.10726. Epub 2020 Sep 30
[PubMed PMID: 33145132]
[84]
Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a Viral Land: A Tale of Two Pathogens. Indian journal of ophthalmology. 2021 Feb:69(2):244-252. doi: 10.4103/ijo.IJO_3774_20. Epub
[PubMed PMID: 33463566]
[85]
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes & metabolic syndrome. 2021 Jul-Aug:15(4):102146. doi: 10.1016/j.dsx.2021.05.019. Epub 2021 May 21
[PubMed PMID: 34192610]
Level 3 (low-level) evidence
[86]
Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. The American journal of emergency medicine. 2021 Apr:42():264.e5-264.e8. doi: 10.1016/j.ajem.2020.09.032. Epub 2020 Sep 16
[PubMed PMID: 32972795]
[87]
Armstrong BK, Murchison AP, Bilyk JR. Suspected orbital myositis associated with COVID-19. Orbit (Amsterdam, Netherlands). 2021 Dec:40(6):532-535. doi: 10.1080/01676830.2021.1962366. Epub 2021 Aug 17
[PubMed PMID: 34402364]
[88]
Eleiwa T, Abdelrahman SN, ElSheikh RH, Elhusseiny AM. Orbital inflammatory disease associated with COVID-19 infection. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2021 Aug:25(4):232-234. doi: 10.1016/j.jaapos.2021.04.002. Epub 2021 May 6
[PubMed PMID: 33965589]
[89]
Martínez Díaz M, Copete Piqueras S, Blanco Marchite C, Vahdani K. Acute dacryoadenitis in a patient with SARS-CoV-2 infection. Orbit (Amsterdam, Netherlands). 2022 Jun:41(3):374-377. doi: 10.1080/01676830.2020.1867193. Epub 2021 Jan 5
[PubMed PMID: 33402004]
[90]
Pérez-Chimal LG, Cuevas GG, Di-Luciano A, Chamartín P, Amadeo G, Martínez-Castellanos MA. Ophthalmic manifestations associated with SARS-CoV-2 in newborn infants: a preliminary report. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2021 Apr:25(2):102-104. doi: 10.1016/j.jaapos.2020.11.007. Epub 2021 Feb 16
[PubMed PMID: 33601042]
[91]
Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z, Liu L, Zhang G, Wang J. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. The British journal of ophthalmology. 2020 Jun:104(6):748-751. doi: 10.1136/bjophthalmol-2020-316304. Epub 2020 Apr 7
[PubMed PMID: 32265202]
Level 2 (mid-level) evidence
[92]
Amesty MA, Alió Del Barrio JL, Alió JL. COVID-19 Disease and Ophthalmology: An Update. Ophthalmology and therapy. 2020 Sep:9(3):1-12. doi: 10.1007/s40123-020-00260-y. Epub 2020 May 22
[PubMed PMID: 32445134]
[93]
Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Musch DC, Mah FS, Dunn SP, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Conjunctivitis Preferred Practice Pattern®. Ophthalmology. 2019 Jan:126(1):P94-P169. doi: 10.1016/j.ophtha.2018.10.020. Epub 2018 Oct 23
[PubMed PMID: 30366797]
[94]
Seah I, Su X, Lingam G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Eye (London, England). 2020 Jul:34(7):1155-1157. doi: 10.1038/s41433-020-0790-7. Epub 2020 Feb 6
[PubMed PMID: 32029919]
[95]
Loon SC, Lun K. SARS: a timely reminder. The British journal of ophthalmology. 2013 Sep:97(9):1217-8. doi: 10.1136/bjophthalmol-2013-303596. Epub 2013 Jun 22
[PubMed PMID: 23793905]
[96]
Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor air. 2007 Jun:17(3):211-25
[PubMed PMID: 17542834]
[97]
Wessels IF, Wessels DA, Zimmerman GJ. The photic sneeze reflex and ocular anesthesia. Ophthalmic surgery and lasers. 1999 Mar:30(3):208-11
[PubMed PMID: 10100255]
[98]
Morley AM, Jazayeri F, Ali S, Malhotra R. Factors prompting sneezing in intravenously sedated patients receiving local anesthetic injections to the eyelids. Ophthalmology. 2010 May:117(5):1032-6. doi: 10.1016/j.ophtha.2009.09.007. Epub 2010 Jan 15
[PubMed PMID: 20079540]
[99]
Jun ISY, Hui KKO, Songbo PZ. Perspectives on Coronavirus Disease 2019 Control Measures for Ophthalmology Clinics Based on a Singapore Center Experience. JAMA ophthalmology. 2020 May 1:138(5):435-436. doi: 10.1001/jamaophthalmol.2020.1288. Epub
[PubMed PMID: 32232466]
Level 3 (low-level) evidence
[100]
Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. The Journal of hospital infection. 2020 Mar:104(3):246-251. doi: 10.1016/j.jhin.2020.01.022. Epub 2020 Feb 6
[PubMed PMID: 32035997]