Continuing Education Activity
Mucocutaneous eruptions are rashes that have both mucous membrane and cutaneous distribution. Differences between the history and physical exam patterns of these rashes and associated inciting etiologies have led to the development of separate names for these mucocutaneous eruptions, such as erythema multiforme (EM), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). Recently, a separate pattern of the mucocutaneous rash pattern in association with Mycoplasma pneumoniae respiratory infections has prompted the proposal for an additional mucocutaneous eruption entity, mycoplasma-induced rash and mucositis (MIRM). This activity reviews the cause and pathophysiology of mycoplasma mucositis and highlights the role of the interprofessional team in its management.
Objectives:
Review the presentation of mycoplasma mucositis.
Summarize the treatment of mycoplasma mucositis.
Describe the differential diagnosis of mycoplasma mucositis.
Explain modalities to improve care coordination among interprofessional team members in order to improve outcomes for patients affected by mycoplasma mucositis.
Introduction
Mucocutaneous eruptions are rashes that have both mucous membrane and cutaneous distribution. Differences between the history and physical exam patterns of these rashes and associated inciting etiologies have led to the development of separate names for these mucocutaneous eruptions, such as erythema multiforme (EM), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). Recently, a separate pattern of the mucocutaneous rash pattern[1][2][3] in association with Mycoplasma pneumoniae respiratory infections has prompted the proposal for an additional mucocutaneous eruption entity, mycoplasma-induced rash and mucositis (MIRM). Canavan et al. proposed three types of MIRM,[4] all of which demonstrate:
- Evidence of “atypical pneumonia” by clinical symptoms (fever, cough) and laboratory evidence of M. pneumoniae infection (elevated M. pneumoniae IgM antibodies, positive cultures or polymerase chain reaction for M. pneumoniae from oropharyngeal or bullae, and/or serial cold agglutinins) AND
- A rash to the mucosa (usually greater than or equal to two sites)
However, the three types of MIRM differ by the characteristics of their cutaneous, non-mucosal rash patterns:
- Classic MIRM has evidence of a. and b. above plus a non-mucosal rash (vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%) and morbilliform eruptions (9%).
- MIRM sine rash has a. and b. above, but there is no significant cutaneous, non-mucosal rash (although there may be a “few fleeting morbilliform lesions, or a few vesicles”).
- Severe MIRM has 1 and 2 (above), although greater than 2 sites have been reported, but the cutaneous rash is extensive with widespread non-mucosal blisters or flat atypical target lesions.
Recognition of MIRM as a clinical entity distinct from other mucocutaneous eruptions may be clinically beneficial as treatment options, disease prognosis, and patient education of MIRM will be different from that of the latter.
Etiology
MIRM is caused by infection with M. pneumoniae. M. pneumoniae infections may produce both pulmonary and extra-pulmonary disease.[5] With respect to its pulmonary manifestation, M. pneumonia is associated with so-called atypical pneumonia. Extra-pulmonary conditions include vasculitides, neurologic, immunologic, thrombotic, and cutaneous.
Epidemiology
In general, MIRM is seen in children and young adolescents with a reported mean age of incidence of 12 years.[4] However, there have been case reports of MIRM in young adults as well.[6][7] Sixty-six percent of the identified cases of MIRM occurred in males.[4]
Pathophysiology
MIRM is believed to be caused by the cloning of B cells with subsequent cutaneous immune complex deposition and formation of compliment. Importantly, this is a different process than EM and SJS/TEN, which are mediated by delayed hypersensitivity reaction and Fas ligand-mediated toxicity,[8] leading to the differentiation of MIRM from other cutaneous reactions.
Histopathology
Whether histopathologic features unique to MIRM exist remains unclear. EM, SJS, and MIRM have similar histopathologic features. These include apoptotic keratinocytes and sparse perivascular dermal infiltrates. The existence of distinct biopsy features enabling histopathologic distinction between these diseases remains controversial. Rzany et al. examined specimens from erythema multiforme, SJS, and TEN, and did not find significant, consistent histologic differences; whereas, Wetter and Camilleri identified histopathologic features in drug-induced SJS that were not present in MIRM or immunization-induced SJS in some specimens examined.[9] SJS may have more necrotic keratinocytes, denser dermal infiltrates, and present with microscopic red blood cell extravasation, pigment incontinence, and parakeratosis as compared to MIRM.
History and Physical
History of present illness may help to differentiate MIRM from other mucocutaneous eruptions, such as EM and SJS/TEN. Nearly all patients with MIRM will have prodromal symptoms, such as fever, cough, and malaise approximately one week before the onset of the rash[4]; whereas, EM may be suggested by a history of herpes simplex infection (oral ulcerations). SJS/TEN, currently considered different spectrums of the same underlying process, is often preceded by a prodrome of fever and upper respiratory infection symptoms but is often disguised by a preceding history of exposure to new medications,[10] such as antibiotics (sulfonamides, beta-lactams), NSAIDs (oxicam), allopurinol, antiepileptics (phenytoin, carbamazepine, oxcarbazepine), and nevirapine.
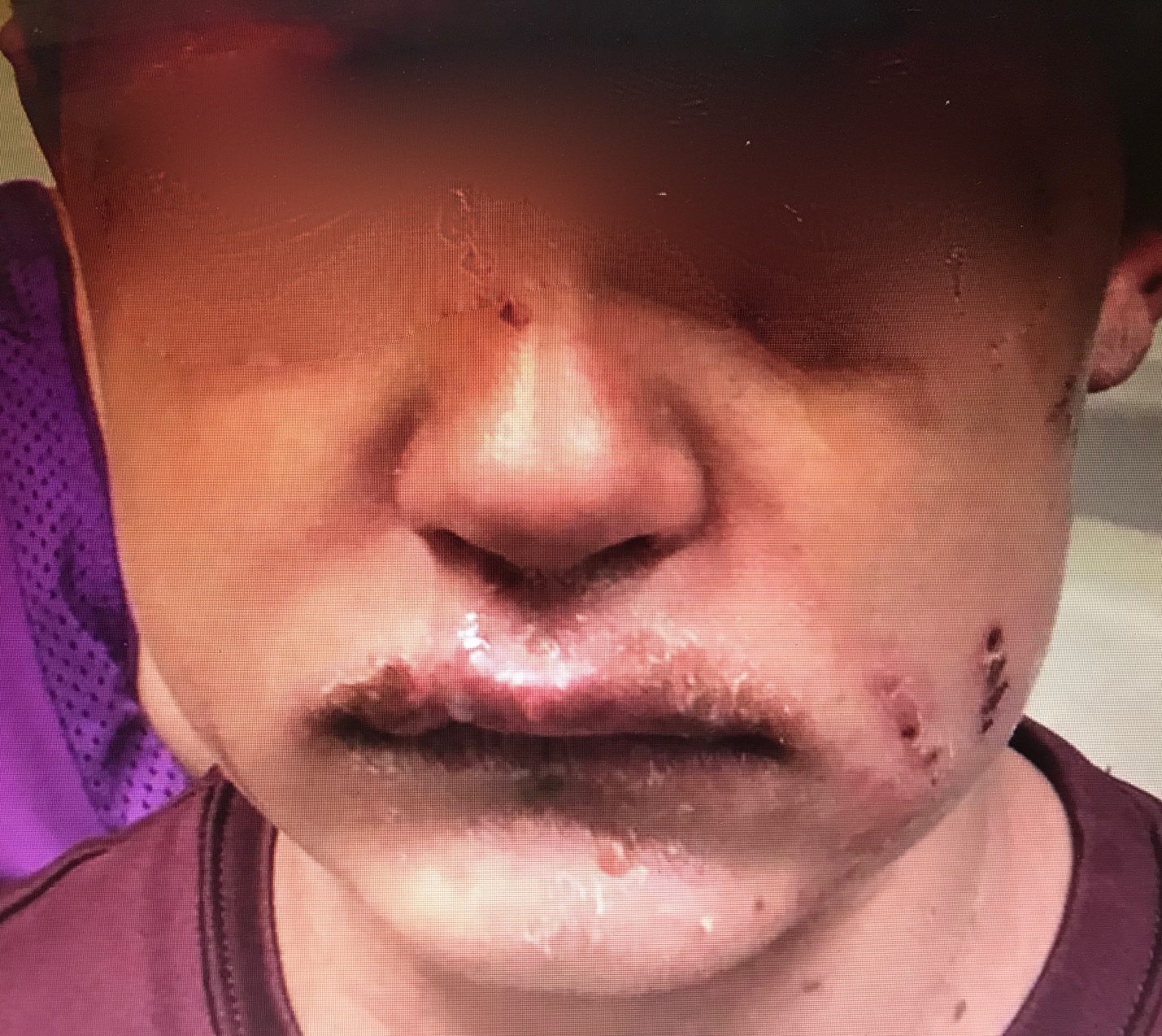
Physical exam findings in MIRM include a predominance of mucosal rashes: oral mucosa (94%), ocular (82%) and urogenital (63%). Other mucosal sites include the nares and anus. Mucosal lesions are typically described as ulcerative or hemorrhagic and may be painful. Nares may demonstrate “dense hemorrhagic crusts,” sometimes manifesting as blood on the tissue. The anus, as part of the GI mucosa, may have lesions that cause pain with defecation.[11]
Additionally, a non-mucosal, cutaneous rash is present in 47%, and it is absent in the remainder of cases and classified as MIRM sine rash. When present, the cutaneous rash of MIRM demonstrates some pattern differences compared to other mucocutaneous eruptions in that MIRM is sparse in overall distribution and, specifically, located more in the acral (limbs) regions (46%) than the trunk (23%). The cutaneous rash morphology is predominantly described as vesiculobullous (77%), as well as both typical target lesions which have three circumferential zones of demarcation, and atypical target lesions which have two color zones (48%). Less commonly, rashes are described as papules (14%), macules (12%), or morbilliform (9%). The amount of skin that is detached is usually less than 10% of the body surface area.
In contrast, EM presents initially as a cutaneous acral rash with macules that evolve into papules, plaques, and subsequently, typical target lesions and/or atypical target lesions (those that are raised). These target lesions spread centripetally to the trunk and face. EM minor has little or no mucous membrane involvement, and EM major has a rash to one or more mucous membranes. SJS/TEN manifests as a rash of macules, purpura, diffuse erythema, atypical target lesions (those that are flat), flaccid blisters that are extensive (not sparse) in number and initially more centrally located and then coalesce and spread to the face and limbs, with extensive mucous membrane involvement to two or more mucosal sites. The amount of skin detachment differentiates the extent of SJS/TEN. SJS has less than 10% skin detachment. Ten to 30% skin detachment is overlap SJS/TEN. Greater than 30% skin detachment is TEN.
Evaluation
Patients diagnosed with MIRM will have pulmonary symptoms that may prompt ordering of a chest radiograph, which may demonstrate evidence of pneumonia.
Results for other laboratory markers in the currently-proposed MIRM diagnosis criteria, such as M. pneumoniae IgM antibodies (elevated), M. pneumoniae in oropharyngeal or bullae cultures or PCR, and/or serial cold agglutinins may not be available in most acute care settings when the diagnosis of MIRM may not yet be established.
Treatment / Management
In the acute care setting, the practitioner may not be able to differentiate MIRM from other mucocutaneous eruptions. Therefore, consultation with an infectious disease specialist or dermatologist or transfer to a facility with the appropriate resources may be considered. All patients with MIRM warrant supportive care, pain control for skin lesions and oral ulcerations (“magic mouthwash” solution or sucralfate), mucosal care, and correction of any fluid and nutritional deficiencies due to decreased oral intake. Severe MIRM with extensive skin detachment may require early transfer to a burn center.[12] Lesions in particular mucosal areas may warrant specialty consultation to ophthalmology, otolaryngology, gastroenterology, and urology to mitigate long-term complications. Currently, there is no evidenced-based pharmacologic treatment paradigm for MIRM. However, patients diagnosed with MIRM often have evidence of atypical pneumonia, and therefore, reasonably benefit from antibiotic treatment.[13] Oral antibiotic options include azithromycin (10 mg/kg on day 1, followed by 5 mg/kg/day once daily on days 2 through 5 to a maximum of 500 mg on day 1, followed by 250 mg on days 2 through 5); oral clarithromycin (15 mg/kg/day in 2 doses to a maximum of 1 g/day); erythromycin, doxycycline for children greater than 7 years old. However, the paucity of data does not indicate if antibiotics instituted early in the course of atypical pneumonia decreases the incidence of MIRM. Additionally, the benefit of immunosuppression therapy in active MIRM is unclear. IVIG may be helpful for MIRM patients with severe mucositis and perhaps for MIRM sine rash. In the Canavan study, 35% of patients received systemic corticosteroids and 8% received IVIG.
Differential Diagnosis
Common differential considerations for MIRM include other conditions that can cause similar skin findings and/or mucosal manifestations[14]:
- Erythema multiforme major
- Toxic epidermal necrolysis/Stevens-Johnsons syndrome
- Drug reaction with eosinophilia and systemic symptoms (DRESS)
- Staphylococcal scalded skin syndrome (SSSS)
- Hand-foot-and-mouth disease
- Kawasaki disease
- Herpetic gingivostomatitis
- Severe cutaneous adverse reactions (SCAR) (e.g., Drug hypersensitivity syndrome)
- Bullous systemic lupus erythematosus
- Plasma cell stomatitis
Prognosis
Overall, the prognosis of MIRM is good. Only 4% of patients required admission to intensive care. Eighty-one percent of MIRM patients recover completely. Overall mortality from MIRM is 3%. Importantly, these cases were reported in the 1940s, which was a pre-macrolide antibiotic era (erythromycin A was first used clinically in 1952).[15] On the other hand, SJS/TEN patients require intensive care more frequently and have higher mortality.[12],[16] The recurrence rate of MIRM is 8%.
Complications
Eighty-one percent of patients with MIRM experience a full recovery. The most commonly reported complications (approximately 11%) involve mucosal sequelae. Ocular mucosal damage occurs in 8.9% and includes conjunctival shrinkage, corneal ulcerations, blindness, ocular synechiae, and loss of eyelashes. Oral (0.8%) and genital (0.8%) mucosal synechiae were also reported. The other commonly reported complication was post-inflammatory pigmentary changes (5.6%). Rare complications include persistent cutaneous lesions, B-cell lymphopenia, restrictive lung disease, and chronic obliterative bronchitis. However, while the mortality of MIRM is 3% (reported in the 1940s), experts suspect that the vast majority of mild cases are underreported in the literature, and the true rate of morbidity and mortality is actually far lower.
Consultations
When the diagnosis of MIRM is being entertained, consultation with infectious diseases specialists or dermatology may be helpful. Consultation with otolaryngology, ophthalmology, gastroenterology, and urology may be obtained for site-dependent mucosal lesions to help mitigate long-term sequelae. Burn specialists should be consulted for extensive cutaneous lesions with detachment.
Deterrence and Patient Education
Patients with MIRM should be advised that mucosal lesions may become painful, limiting oral intake, and resulting in dehydration and lack of nutrient intake. Cutaneous lesions should be kept as clean as possible to avoid secondary bacterial infection. Although patterns of transmission of MIRM are not well established, it is reasonable for patients and close contacts of a suspected M. pneumoniae pulmonary infection to institute basic infection control measures, including droplet transmission precautions.
Pearls and Other Issues
- MIRM is thought to be a clinically distinct entity from other mucocutaneous eruptions.
- Historical features suggesting MIRM include a pulmonary infection approximately one week before the onset of the rash. Other mucocutaneous reactions, such as EM often develops after a history of herpes simplex infection (recent oral ulcers), or the rash of SJS/TEN may occur in the context of new medication use.
- The rash pattern of MIRM may be different than other conditions. Mucous membrane involvement is almost always seen. The cutaneous rash of MIRM is more often sparse, rather than consolidated. It is more often located in acral areas (limbs). The type of rash in MIRM is usually vesiculobullous or target lesions.
- Although MIRM may have some long-term sequelae, such as mucous membrane damage and cutaneous skin changes, MIRM usually has a more benign course compared to other conditions, such as SJS/TEN.
- Treatment of different types of MIRM is not well defined, but patients with active pneumonia reasonably should receive organism directed antibiotics. Consultation with an infectious disease specialist or dermatologist may help to direct care.
Enhancing Healthcare Team Outcomes
MIRM is best managed by an interprofessional team that includes an infectious disease expert, internist, dentist, primary care provider, and even a dermatologist. The key is to educate the patient. An interprofessional team involving the clinician and nurse coordinating care and provide good patient education will result in the best outcomes. [Level V]
Patients with MIRM should be advised that mucosal lesions may become painful, limiting oral intake, and resulting in dehydration and lack of nutrient intake. Cutaneous lesions should be kept as clean as possible to avoid secondary bacterial infection. Although patterns of transmission of MIRM are not well established, it is reasonable for patients and close contacts of a suspected M. pneumoniae pulmonary infection to institute basic infection control measures, including droplet transmission precautions.