Continuing Education Activity
The invasive electrophysiological study (EPS) became a widely used procedure, performed all over the world as an essential part of the diagnosis of arrhythmias. This activity describes the concept, steps, and indications of the electrophysiologic study (EPS) and highlights its utility for patients with different arrhythmia types.
Objectives:
- Identify the clinical picture that indicates the use of EPS.
- Summarize the equipment, personnel, preparation, and technique regarding the EPS.
- Review the indications and clinical significance of the electrophysiologic study.
- Describe some interprofessional team strategies for improving care coordination and communication when using EPS for cardiac arrhythmias.
Introduction
A notable increase in invasive electrophysiological (EP) testing and catheter ablation procedures performed all over the world has been observed over the past twenty-five years. This activity will focus on the indications, technique, and complications of the electrophysiological study (EPS). The EPS is an invasive procedure that needs catheter placement into the right heart via the femoral vein, using the Seldinger technique. The aim is to stimulate the heart using two pacing techniques: extra-stimulus pacing and incremental pacing. The use of extra stimulus pacing reveals the refractory periods, conduction and activation changes, diagnostic for certain diseases. The incremental pacing helps for observing and measuring the impulse conduction during stress conditions and evaluates the recovery time of normal function at the cessation of stimulation.
Anatomy and Physiology
The anatomy of the heart and the landmarks necessary for the EP study require clarification to understand the EP concept and intracardiac signals.
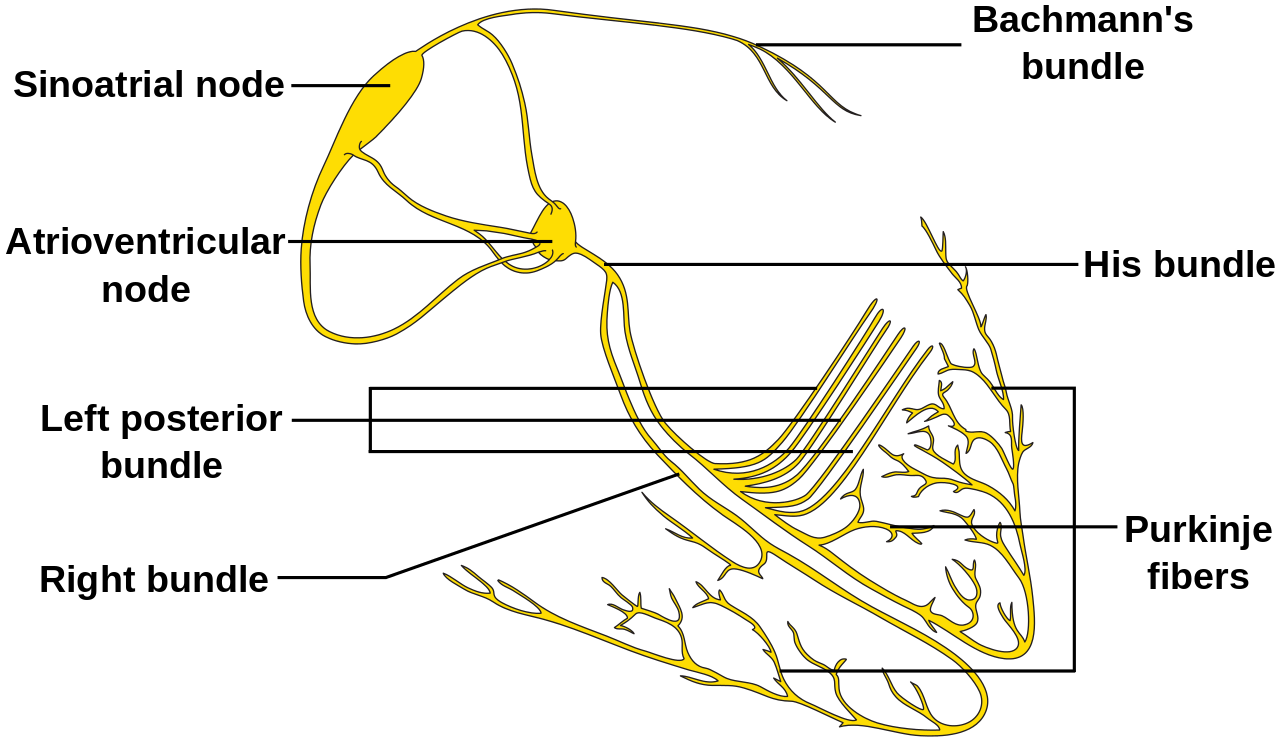
- The right atrium, a structure of great importance for the electrophysiologist, is composed of the appendage, the terminal crest, the body, and the vestibule. This chamber contains a significant part of the electric system of the heart. Crista terminalis is a C shape muscle bundle (ridge) vertically aligned between the right atrium anteromedial wall until the cavotricuspid isthmus. The vertical orientation of the muscle in the terminal crest and the oblique orientation of the rest of the fibers of the right atrium constitute a substrate for arrhythmias (reentry mechanism).
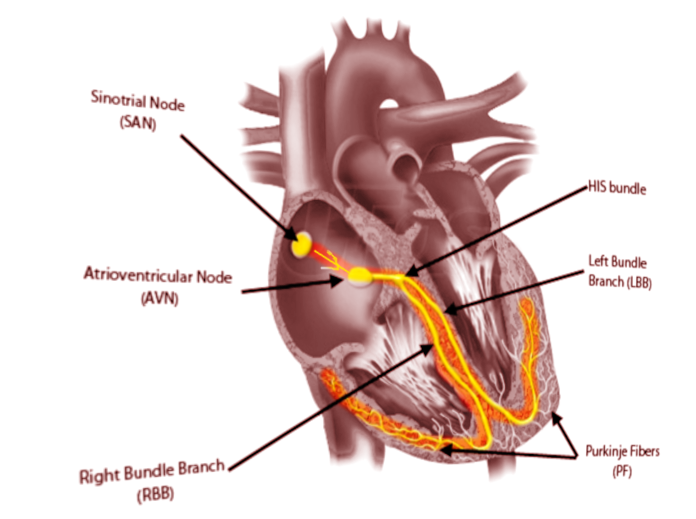
- The sinoatrial node (SN) is a crescent-shaped structure that is part of the electric or excito-conductory system of the heart. It has a superior and anterolateral position at the junction of the RA with the inferior vena cava. The SN gets irrigated by the sinus node artery with an RCA origin in 55% of individuals and a circumflex artery origin in 45%.[1] The SN is a structure with pacemaker function (60 to 100 impulses/minute at rest), a tissue without contractile function which automatically generates electrical impulses, located in the right atrium at the junction with the superior vena cava. Its pacemaker potential, depolarization, and repolarization are dependent on three ion channels for sodium, potassium, and calcium.
- The atrioventricular node (AVN): The impulse arising from the sinoatrial node gets conducted at the atrioventricular node, a relay station between the atria and the ventricle. The AVN is located just at the apex of the Koch triangle, in the paraseptal myocardium. It has a potential pacemaker activity with 20 to 60 impulses per minute (in the absence of the primary pacemaker activity-the SAN). The representative aspect of the AVN is that it presents two conduction pathways - the fast pathway (FP) and the slow pathway (SP). The two pathways can entertain the most common form of supraventricular tachycardia named atrioventricular nodal reentrant tachycardia (AVNRT), always induced by premature beats.
- The Koch triangle. It is an area of the right atrium that contains the atrioventricular node (AVN). The borders of the triangle are in posterior the tendon of Todaro (a fibrous continuation of the Eustachian ridge), inferiorly the coronary sinus ostium with the adjacent vestibulum and anterior the annulus of the tricuspid valve.[2] The fast pathway of the AVN is at the apex of the triangle of Koch. The dimensions of the triangle can vary a lot from an individual to another. This finding is of great importance during mapping and ablation procedures of structures contained within this area (usually the slow pathway a part of the atrioventricular nodal reentry tachycardia circuit).[3]
- The cavotricuspid isthmus. It is an inferior area of the RA lying between the inferior vena cava and the tricuspid valve, which conventionally and anatomically divides into three parts: inferolateral inferior and paraseptal. The CTI is essential in the genesis and maintenance of the typical isthmus-dependent atrial flutter.[4] In one fifth to one-third of the population, the CTI is U shaped or has a recess (also named sub-Eustachian sinus, sinus of Keith, or sub-Thebesian recess) that makes mapping and catheter ablation difficult.
- The Eustachian valve is a flap with a crescent shape that guards the inferior vena cava at the junction with the RA. Sometimes this valve is large and may impede the access of the catheters into the RA.
Indications
Electrophysiologic testing is indicated in patients with different arrhythmias. Even the genesis of arrhythmia is more complicated; it can be summarized in three significant mechanisms: automaticity, triggering, and reentry. Most arrhythmias are reentrant, necessitating a critical isthmus to maintain.
Indications of the EPS
- Supraventricular tachycardia (SVT)The SVT or narrow complex tachycardia is frequent and associated with a heart rate above 100/minute and a QRS complex shorter than 120ms.
- Narrow QRS complex SVT Narrow QRS tachycardia that usually needs EPS: regular narrow QRS tachycardias- sinus tachycardia, inappropriate sinus tachycardia, sinus nodal reentrant tachycardia, focal atrial tachycardia, atrial flutter with stable atrioventricular conduction, atrioventricular nodal reentrant tachycardia, junctional ectopic tachycardia, orthodromic atrioventricular reentrant tachycardia and irregular narrow QRS tachycardias- atrial fibrillation, focal atrial tachycardia with variable atrioventricular block, atrial flutter with variable atrioventricular block, multifocal atrial tachycardia. EPS should be performed to diagnose SVT, particularly when anticipating catheter ablation. Because of the narrow QRS complex, a high septal ventricular tachycardia can mimic an SVT. Accessory pathways- the majority of accessory pathways conduct both anterogradely and retrogradely. The 2019 ESC Guidelines (the first guideline update in 16 years) regarding the management of patients with SVT recommends (class I) EPS in asymptomatic patients (including athletes and patients with high occupational risk) for assessment of sudden cardiac death risk. Catheter ablation is indicated when EPS with the use of isoprenaline identifies high-risk features: anterograde effective refractory period below or equal to 250ms, RR minimal pre-excited interval during atrial fibrillation less than or equal to 250 ms, multiple accessory pathways, induction of an accessory pathway-dependent tachycardia.[5]
- Wide QRS complex SVT- regular (SVT with an QRS complex over 120 ms): antidromic atrioventricular reentrant tachycardia, all supraventricular tachycardias with aberration or preexisting or rate-dependent bundle branch block, atrial tachycardia with preexcitation having the accessory pathway as a bystander and irregular: atrial fibrillation, atrial flutter or tachycardia with variable atrioventricular block, conducted with aberration, antidromic atrioventricular tachycardia due to a nodo-ventricular or nodo-fascicular accessory pathway with variable ventriculoatrial conduction. The EPS in those patients aims to establish the underlying mechanism and to guide catheter ablation.
- Ventricular tachycardia (VT)
- Non-sustained VTs. The absence of structural heart disease, non-sustained VT, is not considered a risk marker for sudden death. The situation changes dramatically when a structural cardiac disease and LV failure is present because in association with non-sustained VT results in a negative prognostic. The EPS is indicated in symptomatic patients with non-sustained VT to identify potential candidates for radiofrequency ablation or ICD implantation (class II).
- Sustained monomorphic VTs. Patients with structurally normal hearts the most frequent VT is the right ventricular outflow tract VT. An EPS should take place before catheter ablation in those patients. In patients with structural heart disease, the most common underlying mechanism associated with VT during or after the healing of myocardial infarction is reentry. Two essential conditions must occur for reentry- successful conduction in one direction only (unidirectional block) and a circuit cycle length superior to any effective refractory period of the structures implicated into the circuit. The clinical tolerance of a ventricular tachycardia relates to the heart rate, retrograde conduction through the AVN, the basal ejection fraction of the left ventricle, and other compensatory mechanisms. The EPS is a vital investigation mainly because it identifies patients at risk for sudden cardiac death, identifies those patients eligible for ICD implantation, and guides catheter ablation (class I). It is useful to assess VT recurrence under chronic antiarrhythmic treatment and to guide the catheter ablation in case of recurrence. The ICD implantation is recommended as the first intention in several cases: the patient has multiple episodes of VT, which are hemodynamically not tolerated, the patient is receiving chronic optimal antiarrhythmic treatment at the moment of multiple episodes of a stable VT, and the ejection fraction is under 35%.[6]
- Polymorphic VTs- is an unstable rhythm with continuously changing QRS morphology. It is usually associated with the acute setting of the myocardial infarction, but it can present in association with cardiomyopathies (hypertrophic cardiomyopathy, dilated cardiomyopathy) and other channelopathies (Brugada syndrome, short QT syndrome, and catecholaminergic polymorphic VT). The EPS is not routinely recommended in patients with acute myocardial infarction. However, it might play a role in patients with inherited diseases. In patients with hypertrophic cardiomyopathy, the EPS can guide radiofrequency ablation of associated arrhythmia like SVT and preexcitation (class I) and to identify the indication for catheter ablation in symptomatic patients with monomorphic, sustained VT. In patients with arrhythmogenic right ventricular dysplasia, the EPS is considered for sudden cardiac risk stratification (class II).
- Premature ventricular contractions. The current consensus specifies that isolated PVC with a structurally normal heart is considered benign. In patients with structural heart disease and left ventricular dysfunction, more than 10 PVCs/hour and runs of non-sustained VT represent a marker of increased risk for sudden death. More than 24% PVC per 24h and a short coupling interval < 300ms or R on T phenomenon are also markers of risk. EPS is necessary in the case of symptomatic PVCs associated with increased markers of sudden death to guide radiofrequency ablation.
- Sinus node dysfunction includes sinus bradycardia, sinoatrial block, sinus arrest, chronotropic incompetence, sinus pause. The EPS is used to assess the link between the symptoms and the sinus node dysfunction and sinus bradycardia in which non-invasive methods cannot establish a direct relation (class I).
- Conduction abnormalities.
- Atrioventricular block. Symptomatic patient associated with the entire situations when the non-invasive diagnostic fail to specify the level of block.
- Bundle branch block/ /bifascicular block/trifascicular block/ intraventricular conduction disease.
- Bifascicular block is classically one of the two following variants: right bundle branch block (RBBB) + left anterior hemiblock (LAHB) or RBBB + left posterior hemiblock(LPHB)
- Trifascicular block is one of the three following variants: alternation of RBBB+ LAHB and RBBB+LPHB, alternation of LBBB and RBBB or combination of bifascicular block and a type I atrioventricular block with an infrahisian location
- Left bundle branch block (LBBB) can be considered as a variant of bifascicular block. EPS performed in this patient aims to identify the patient at risk of a complete atrioventricular block and the need for pacemaker implantation.
- Nonspecific intraventricular conduction disease is a prolongation of the QRS complex, which does not accomplish the criteria of LBBB or RBBB.
- Syncope-patients with unexplained syncope, bi-fascicular bundle branch block, and an HV interval greater than or equal to 70 ms determined during the EPS, the implantation of a pacemaker is recommended (class I, level of evidence B) according to the 2018 ESC Guidelines on diagnosis and management of syncope. An EPS is indicated when syncope remains unexplained after non-invasive evaluation in patients with syncope and previous myocardial infarction or other scar-related conditions (class I, level of evidence B). Whereas a positive EPS predicts the cause of syncope, a negative study cannot exclude an arrhythmic syncope; therefore, further evaluation is necessary. EPS is generally useful in patients with syncope, abnormal ECG, structural heart disease, and palpitations.[7]
- Other indications for EPS include patients with progressive cardiac conduction disease, dilated cardiomyopathy, muscular dystrophies (Duchenne, Becker), post antiarrhythmic surgery, sarcoidosis, congenital heart disease, survivors of cardiac arrest, undocumented palpitations, guiding drug therapy, conduction disorders after transcatheter aortic valve replacement.[8]
Personnel
An EPS requires a specialized team composed of one or two electrophysiologists, a technician, and one or two nurses. The electrophysiologist usually manipulates the catheters; the technician is operating the external stimulator, the data acquisition system, and the ablation generator, and the nurse takes direct care of the patient in terms of preparation, hemodynamic status, and pulse-oximetry monitoring, administration of drugs and oxygen.
Preparation
The EPS is an invasive diagnostic procedure. Discontinue antiarrhythmic therapy for at least five half-lives before the procedure. The patient needs to be after a minimal period of 6 hours of fasting. Anticoagulation therapy should also be discontinued, and hypoglycemic drugs adjusted for the fasting period. One or two peripheral venous access are necessary before the patient arrives in the EP laboratory. Once in the laboratory, surface electrocardiogram (ECG) electrodes are attached to obtain a standard 12 leads ECG integrated into the EP acquisition system. Non-invasive arterial pressure monitoring by a standard blood pressure cuff-monitor is needed. Permanent pulse-oximetry monitoring is via a standard pulse-oximeter. As the classical 12 lead surf ECG provides a significant amount of information about the heart's electrical activity recorded from twelve different perspectives corresponding to each of the twelve leads, each of the intracardiac diagnostic catheters collects information from inside the heart in terms of position, timing, and voltage.
Technique or Treatment
For a standard EPS, a standard number of four catheters is necessary. Depending on the center, the operator can also use three diagnostic catheters during EPS. Every diagnostic catheter has two or multiple electrodes, and for each pair of consecutive electrodes, a distinct intracardiac electrogram gets recorded.[9] Standard intracardiac electrograms are collected and recorded from the high right atrium, His bundle, the apex of the right ventricle, and coronary sinus. [10] The coronary sinus, a cardiac vein with a posterior trajectory over the left atrioventricular junction, with a diameter that permits the insertion of a diagnostic catheter, is an essential structure. Multiple electrodes (usually decapolar) deflectable catheter inserted in the coronary sinus collects simultaneous electrograms of good quality and amplitude from the left atria and left ventricle.
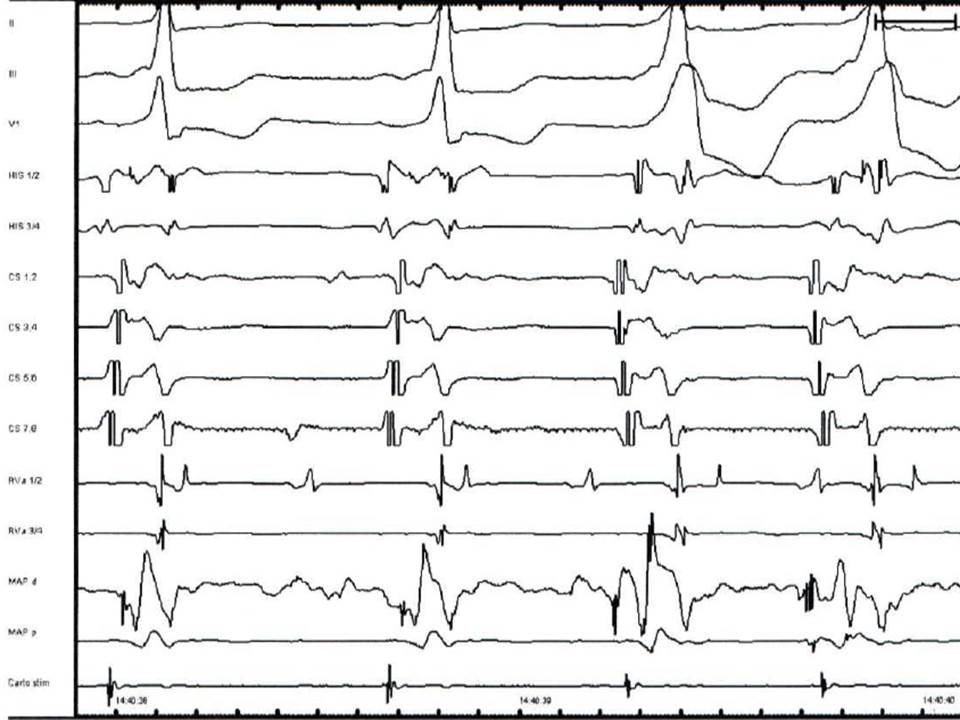
Fig.1. Electrograms displayed during standard four-catheter EPS in sinus rhythm. Three surface ECG leads display results (I, III, V1). ODH - a bipolar catheter records high right atrium electrogram; HIS- atrial electrogram, His electrogram and ventricular electrogram displayed simultaneously and recorded from the AV junction by a quadripolar or hexapolar catheter, VD- electrogram recorded from the right ventricular apex by a bipolar diagnostic catheter, SCP- proximal coronary sinus, SCM- medium coronary sinus, SCD-distal coronary sinus; all the coronary sinus bipoles record a sharp atrial electrogram and a smaller ventricular electrogram- usually recorded by a hexapolar or decapolar catheter.
Measurements and stimulation protocols
The specific intervals measured during sinus rhythm are:
- PA- time for activation to travel into the right atrium between the region of the sinus node and the region of the atrioventricular node. This interval gets measured between the P wave onset and atrial electrogram recorded by the His bundle catheter. The standard value is considered 25 to 55 ms
- AH- time for activation to travel over the AVN; is measured between atrial electrogram recorded on His bundle catheter and His electrogram. The normal value is 55 to 125 ms
- H- the duration from the beginning to the last component of the His bundle electrogram. The standard value is under 30 ms
- HV- is the conduction time over the specialized ventricular conduction system; it gets measured between the His bundle electrogram and the earliest ventricular activation. The standard value is 35 to 55ms.
Sinus node recovery time (SNRT)- is time taken for the sinus rhythm to resume after 30 seconds of overdrive atrial pacing at several cycle lengths (800, 700, 600, 500, 450, 400 and 350 ms). The normal values of the SNRT must be less than 1500ms. The corrected SNRT is the difference between the SNRT and the sinus cycle length; the normal value is below 550 ms.
Atrial extra-stimulus testing consists of a drive train of 8 paced beats at a constant cycle length (600 and 400ms) followed by an extra-stimulus delivered on the same catheter, on the same site. The drive train and the extra stimulus with a progressive decrescent value are repeating until there is a loss of atrial capture. The atrial extra stimulus testing serves for evaluation of the anterograde conduction over the AVN, atrial refractoriness, and induction of specific arrhythmias.
Ventricular extra-stimulus testing is similar to the atrial ventricular pacing. The stimulation is made at the right ventricular apex conventionally. Ventricular extra-stimulus testing is used for the evaluation of the retrograde conduction over the atrioventricular node (concentric atrial activation), presence of the accessory pathways (eccentric activation), ventricular refractoriness, and induction of specific arrhythmias. Multiple extra-stimuli are available for arrhythmia induction.
Incremental atrial pacing supposes stimulation on the high atrial catheter at a cycle length that progressively decreases. Between each decreasing step of the stimulated cycle length, the rhythm is observed several seconds. One of the most valuable observations during this pacing protocol is the Wenkebach cycle length, the stimulated cycle at which the 1:1 atrioventricular conduction over the atrioventricular node stops.
Incremental ventricular pacing is similar to the ventricular pacing. The pacing is delivered from the right ventricular apex. The minimum cycle length of ventricular pacing is 300ms. The observations during incremental ventricular pacing are the assessment of the retrograde Wenkebach cycle length and the pattern of retrograde atrial activation.
Pacing at other sites than the usual ones (high right atrium and ventricular apex) may reveal the accessory pathways. The use of isoproterenol and atropine is widely used for tachycardia induction during the EPS.
Enhancing Healthcare Team Outcomes
As many of the challenging arrhythmic events leading to sudden cardiac death occur in athletes; a good collaboration between interprofessional team members from sports medicine and cardiologist is necessary. However, the complex adaptations induced by exercise present a continuous challenge to the cardiologist asked to evaluate athletes. A unique discipline of sports and exercise cardiology tailoring cardiovascular care in athletes and exercising individuals would be a fabulous resort in the future, allowing safe participation in sports or enhanced physical activity.[11] A patient safety program to design and implement a system that takes into account the concerns of the frontline personnel requires development.[12] An arrhythmia team with medical co-leads with specific responsibilities and roles formed by electrophysiologists and cardiovascular surgeons constitutes the future of the therapeutic strategy regarding complex patients. Besides, the arrhythmia team should include a general cardiologist, a cardiac anesthetist, an intensivist, a nurse coordinator, and not least a patient representative.[13]
A cardiology specialty nurse can assist with setting up the procedure and answering the patient questions, as well as helping with whatever interventions may be necessary based on the outcome of the EPS, which places nursing as a vital component of the interprofessional team, leading to better patient outcomes. [Level 5]