Continuing Education Activity
Cerebral palsy is a group of permanent disorders affecting the development of movement and causing a limitation of activity. Non-progressive disturbances that manifest in the developing fetal or infant brain can lead to cerebral palsy; this is the most common cause of childhood disability. The degree and type of motor impairment and functional capabilities vary depending on the etiology. Cerebral palsy may have several associated comorbidities, including epilepsy, musculoskeletal problems, intellectual disability, feeding difficulties, visual abnormalities, hearing abnormalities, and communication difficulties.
This activity comprehensively reviews the evaluation, treatment, and complications of cerebral palsy. Managing cerebral palsy should employ an interprofessional approach, and this course underscores the importance of interprofessional team collaboration. Participants will explore strategies tailored to address associated comorbidities, fostering improved patient outcomes through collaborative teamwork across disciplines.
Objectives:
Evaluate the pre-, peri-, and post-natal etiologies that can result in cerebral palsy.
Identify the physical examination process to evaluate a patient who presents with cerebral palsy.
Screen patients within the interprofessional team to manage cases of cerebral palsy.
Implement interprofessional team strategies to improve outcomes in patients diagnosed with cerebral palsy.
Introduction
Cerebral palsy is a group of permanent disorders affecting movement development and limiting activity. Non-progressive disturbances that manifest in the developing fetal or infant brain can lead to cerebral palsy.[1] This palsy is the most common cause of childhood disability. The degree and type of motor impairment and functional capabilities vary depending on the etiology.
The multifactorial etiology relates to prenatal, perinatal, or postnatal causes. The palsy may have several associated comorbidities, including epilepsy, musculoskeletal problems, intellectual disability, feeding difficulties, visual abnormalities, hearing abnormalities, and communication difficulties. Several subtypes warrant consultation from a neurologist if early signs consistent with family history are evidenced. Once diagnosed, holistic care is required to maintain quality of life. As such, treating cerebral palsy requires an interprofessional approach, given the immense burden on functional status.
Etiology
Abnormal development or damage to the fetal or infant’s brain causes cerebral palsy. The brain insult/injury causing cerebral palsy is non-progressive (eg, “static”) and can occur in the prenatal, perinatal, or postnatal periods. The etiology of an individual patient is often multifactorial.[2][3]
Prenatal Causes
- Congenital brain malformations
- Intrauterine infections
- Intrauterine stroke
- Chromosomal abnormalities
Perinatal Causes
- Hypoxic-ischemic insults
- Central nervous system (CNS) infections
- Stroke
- Kernicterus
Postnatal Causes
- Accidental and non-accidental trauma
- CNS infections
- Stroke
- Anoxic insults
Prematurity is a significant risk factor for cerebral palsy. Complications of prematurity that can cause cerebral palsy include:
- Periventricular leukomalacia
- Intraventricular hemorrhage
- Periventricular infarcts [2][3]
Other risk factors associated with cerebral palsy are multiple gestation, intrauterine growth restriction, maternal substance abuse, preeclampsia, chorioamnionitis, abnormal placental pathology, meconium aspiration, perinatal hypoglycemia, and genetic susceptibility.[4][5]
Epidemiology
Cerebral palsy is the most common cause of childhood disability and occurs in 1.5 to 2.5 per 1000 live births.[6] The prevalence is significantly higher in infants born prematurely than infants born at term. The risk of developing cerebral palsy increases with declining gestational age, with infants born at less than 28 weeks gestational age being at the most risk.[6] The prevalence is also higher in infants with low birth weight (less than 1500 g), who are at the most significant risk; 5% to 15% of infants born weighing less than 1500 g develop cerebral palsy.[6] Prenatal events cause approximately 80% of cerebral palsy cases, and postnatal events cause about 10% of cases.
History and Physical
Cerebral palsy is a clinical diagnosis based on information gathered from the patient’s history and physical exam. The clinical history should focus on identifying risk factors and likely etiologies of the patient’s cerebral palsy. The history should include a detailed prenatal, birth, and developmental history. Developmental history should pay particular attention to motor development, as this delay is commonly seen in cerebral palsy. A history of developmental regression is not consistent with cerebral palsy; family history is important as well. When several family members share characteristics of delayed development or neurological disorders akin to the patient’s, it is essential to consider a genetic cause for cerebral palsy or conditions that mimic its symptoms.[7][8] Clinical history should also focus on screening for comorbid disorders, including epilepsy, musculoskeletal abnormalities, pain, visual and hearing difficulties, feeding problems, communication disorders, and behavioral disorders.
The physical exam should focus on identifying clinical signs of cerebral palsy. Head circumference, mental status, muscle tone and strength, posture, reflexes (primitive, postural, and deep tendon reflexes), and gait should undergo evaluation. Clinical signs and symptoms of cerebral palsy can include micro- or macrocephaly, excessive irritability or diminished interaction, hyper- or hypotonia, spasticity, dystonia, muscle weakness, the persistence of primitive reflexes, abnormal or absent postural reflexes, incoordination, and hyperreflexia.
The physical exam can also identify the cerebral palsy type. Cerebral palsy characteristically demonstrates the kind of tone abnormality and distribution of motor abnormalities. The subtypes of cerebral palsy are:
- Spastic diplegic: The patient has spasticity and motor difficulties affecting the legs more than the arms.
- Spastic hemiplegic: The patient has spasticity and motor difficulties affecting one side of the body; the arms are often involved more than the legs.
- Spastic quadriplegic: The patient has spasticity and motor difficulties affecting all 4 extremities; often, a greater involvement of the upper extremities is seen compared to the legs.
- Dyskinetic/hyperkinetic (choreoathetoid): The patient has excessive, involuntary movements characterized as a combination of rapid, dance-like contractions of muscles and slow writhing movements.
- Dystonic: The patient has involuntary, sustained muscle contractions causing twisting and repetitive movements.
- Ataxic: The patient has unsteadiness and incoordination, and they are hypotonic.[9]
Evaluation
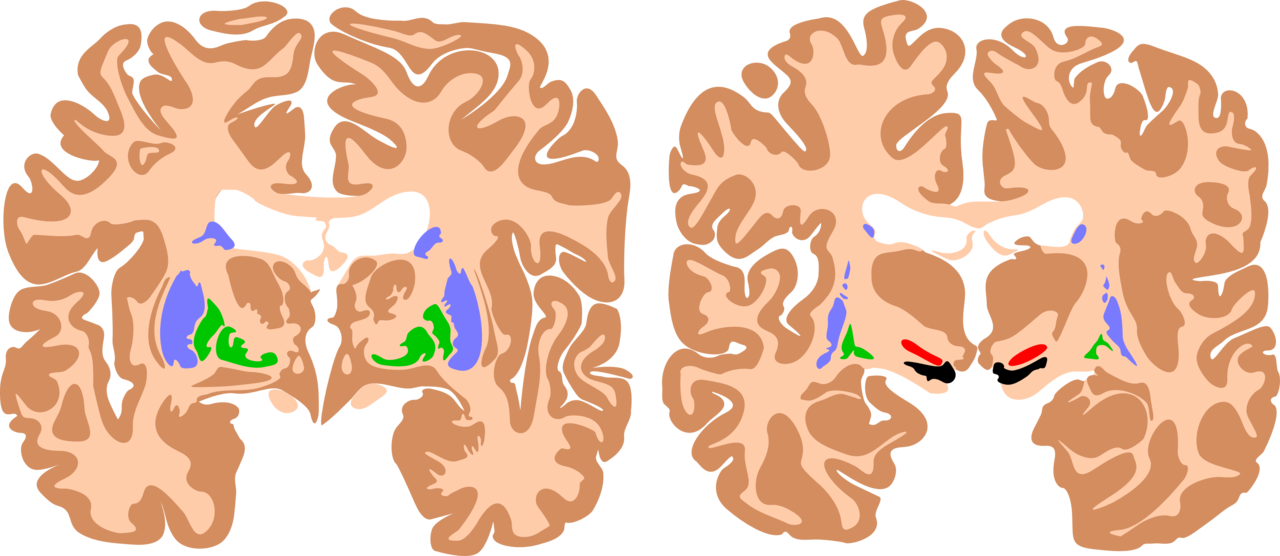
Clinical history, physical exam, neuroimaging, and standardized developmental assessments are useful in diagnosing cerebral palsy. Brain magnetic resonance imaging (MRI) is the preferred imaging modality for evaluating the cause of cerebral palsy (see Image. Basal Ganglia, Cerebral Palsy Brain). MRI has a higher diagnostic yield than computed tomography and provides better detail of the brain’s anatomy. MRI has an 86% to 89% sensitivity for detecting abnormal neuroanatomy in the brain’s motor areas.[10] A cranial ultrasound performed in the neonatal/early infancy period can be useful in identifying intraventricular hemorrhage, ventriculomegaly, and periventricular leukomalacia.
Standardized developmental assessments and neuroimaging should be used for early detection of cerebral palsy. The General Movements Assessment (GM) is a standardized motor assessment used in children younger than 5 months.[10] The GM observes the quality of spontaneous movements in infants while lying supine. Cramped-synchronized general movements and the absence of fidgety movements between 9 and 20 months reliably predict cerebral palsy and have a 98% sensitivity and 89% to 93% inter-rater reliability.[10] The Hammersmith Infant Neurological Exam (HINE) is a standardized neurological assessment for children between 2 and 24 months. HINE consists of 37 items subdivided into 3 sections: physical exam, motor development documentation, and behavior state evaluation. The HINE has a 90% sensitivity for detecting cerebral palsy.[10]
An electroencephalogram is necessary for patients suspected of having seizures. Patients with stroke as a cause of their cerebral palsy should undergo thrombophilia screening. Pro-thrombotic coagulation abnormalities are present in 50% to 60% of patients with a history of stroke.[8]
The clinical signs and symptoms of cerebral palsy can occur in many other conditions. Slowly progressive disorders can be mistaken for cerebral palsy, and sometimes, these disorders have a specific treatment that halts the progression, prevents complications, or treats the primary underlying pathophysiology of the condition. Screening for disorders that mimic cerebral palsy when the clinical history, physical exam, and neuroimaging are atypical for cerebral palsy is critical. The following historical features are concerning for an alternative diagnosis:
- Family history of cerebral palsy or other neurologic disorders
- No known risk factor for cerebral palsy
- Developmental regression
- Hypotonia associated with weakness
- Rapid loss of neurologic skills, worsening during fasting or illness
- Oculomotor abnormalities
- Sensory loss [8][11]
A metabolic workup to screen for inborn errors of metabolism is necessary for patients with a progressive course or decompensation during periods of catabolism.[11] Genetic workup is recommended for those with dysmorphic features, brain malformations, a family history of cerebral palsy, or a history of consanguinity. Lumbar puncture should be obtained in patients with unexplained refractory seizures or movement disorders to screen for neurotransmitter disorders and glucose transporter deficiency.[8][11]
Treatment / Management
Treating cerebral palsy requires an interprofessional team approach, including physicians (primary care, neurologists, physiatrists, orthopedists, and other specialists needed based on co-existing conditions), therapists (physical, occupational, and speech), behavioral health specialists, social workers, case managers, and educational specialists. Interventions should maximize the quality of life and decrease the disability burden. The patient, family, and team should set functional goals that are realistic and periodically reevaluated.[10]
Oral and injectable (eg, botulinum toxin) medications can help treat tone abnormalities, pain, and comorbid conditions such as epilepsy, sialorrhea, gastrointestinal disturbances, and behavior disorders. Medications for spasticity include benzodiazepines, baclofen, dantrolene, tizanidine, cyclobenzaprine, botulinum toxin, and phenol.[12] Clinicians often treat dystonia with trihexyphenidyl, gabapentin, carbidopa-levodopa, and benztropine. Sialorrhea treatment includes glycopyrrolate, atropine drops, and scopolamine patches. Anti-seizure medications are used in patients with epilepsy. Constipation is a frequent complication of cerebral palsy, requiring stool softeners and pro-motility agents. Anti-inflammatories address pain, and antidepressants are used for depression and anxiety. Surgical management options include placement of a baclofen pump, selective dorsal rhizotomy, tendon releases, hip derotation/rotation surgery, spinal fusion, strabismus repair, and deep brain stimulation.[12][13]
Differential Diagnosis
Conditions that can mimic cerebral palsy include neurodegenerative disorders, inborn errors of metabolism, developmental abnormalities of the spinal cord, neuromuscular disorders, movement disorders, and neoplasms. Below is a list of differential considerations based on the predominant clinical feature, including:
Spasticity
- Hereditary spastic paraplegia
- Tethered cord
- Spinal cord tumor
- Adrenoleukodystrophy
- Arginase deficiency
- Pyruvate dehydrogenase deficiency
- Rett syndrome
- Lesch-Nyhan syndrome
- Pelizaeus-Merzbacher
- GLUT-1 transporter deficiency
Dystonia
- Dopa-responsive dystonia
- Glutaric aciduria type 1
- Pyruvate dehydrogenase deficiency
- Lesch-Nyhan syndrome
- Leigh disease
- Niemann-Pick type C
- GLUT-1 transporter deficiency
Hypotonia
- Holocarboxylase synthetase deficiency
- Zellweger syndrome
- Infantile Refsum disease
- Pontocerebellar hypoplasias
- Metachromatic leukodystrophy
Ataxia
- Ataxia-telangiectasia
- X-linked spinocerebellar ataxia
- Angelman syndrome
- GLUT-1 transporter deficiency
- Leigh disease
- Joubert syndrome
Choreoathetosis
- Pelizaeus-Merzbacher
- Lesch-Nyhan syndrome
Weakness
- Muscular dystrophies
- Metachromatic leukodystrophy
- Pontocerebellar hypoplasias [3][7][11]
Prognosis
Most children with cerebral palsy will survive into adulthood.[14] Severely affected patients have a reduced life expectancy. The most common cause of early death is a respiratory disease, usually aspiration pneumonia. The prognosis of motor abilities depends on the cerebral palsy subtype, the rate of motor development, ascertainment of developmental reflexes, and cognitive skills. Children who walk independently typically achieve this milestone by 3 years of age. Those who walk with support may take up to age 9 years to reach this milestone.[15] A child who is not walking by the age of 9 years is unlikely to walk with support. Children with hemiplegic, choreoathetoid, and ataxic cerebral palsy are likely to achieve walking. Good prognostic indicators for independent walking are sitting by the age of 24 months and crawling by 30 months.[15] Poor prognostic indicators for walking include not achieving head balance by 20 months, having primitive reflexes retained, having no postural reflexes by age 24 months, and not crawling by 5 years.[15]
Complications
A variety of complications can accompany cerebral palsy, including:
- Pain: 50% to 75%
- Intellectual disability: 50%
- Epilepsy: 25% to 45%
- Orthopedic disorders (hip subluxation/dislocation [30%], foot deformities, and scoliosis)
- Speech impairment: 40% to 50%
- Hearing impairment: 10% to 20%
- Blindness: 10%
- Strabismus: 50%
- Neurobehavioral disorders: 25%
- Growth failure
- Pulmonary disease
- Osteopenia: 77% of those moderate-severely affected
- Urologic conditions (incontinence, neurogenic bladder): 30% to 60%
- Sleep disturbances: 23%
- Dental abnormalities [9]
Postoperative and Rehabilitation Care
Research results have shown this population benefits from cardio training (especially rhythmic training), with frequency, intensity, time, and type contingent on factors such as baseline fitness levels, support available, and goals. In the past, muscle strengthening was believed to be avoided in individuals with spasticity because it was thought to worsen the condition. Still, results from recent research have shown the benefits of strength training and targeted strength training as a possible means to reduce spasticity (continued research is needed to validate these findings further). Preferred exercises are typically multi-joint (or functional) exercises. However, single-joint exercises can also be utilized, particularly for significantly deconditioned individuals or those who tend to significantly compensate when completing multi-joint exercises.[16]
Gait training has been documented as the most effective way to improve gait speed (compared to other methods, including strength, velocity, and biofeedback). This training should be used appropriately, as gait speed is a significant indicator of overall quality of life and functional mobility.[17] Passive stretching has historically been utilized to reduce spasticity (with various forms of stretching utilized); however, research results have displayed limited data to support passive stretching for a range of motion or functional benefits. According to the literature, prolonged stretches are of greater benefit, but continued research is needed to determine the impact of stretching.[18] Family-centered practices have been established as necessary when completing therapy, as research results show a notable improvement in outcomes when therapies are family-centered. Collaboration on the care plan with the patient and their support system is critical.[19]
Deterrence and Patient Education
Cerebral palsy is a term used to describe a group of disorders caused by a non-progressive brain abnormality that results in difficulty with movement, tone, or posture. Cerebral palsy is the most common cause of childhood disability; several factors during pregnancy, around the time of birth, and after birth play a role in the development of cerebral palsy. The significant risk factors for cerebral palsy are prematurity and low birth weight. Other causes of cerebral palsy include stroke, lack of oxygen to the brain, infections of the brain, and abnormal development of the brain. Cerebral palsy is a clinical diagnosis by obtaining a detailed prenatal and birth history, physical exam, and neuroimaging. Treatment focuses on achieving the best functional outcomes and takes an interprofessional team approach—routine prenatal care and measures to reduce preterm birth and lower the risk of cerebral palsy.
Enhancing Healthcare Team Outcomes
Clinicians should recognize the signs of cerebral palsy and make the diagnosis of “high risk” as early as possible. Historically, the diagnosis of cerebral palsy was made between the ages of 12 to 24 months. No good evidence supports that the diagnosis can be accurately predicted before 6 months of age at a corrected age.[10] Early diagnosis can improve functional outcomes and reduce disease burden because early interventions can optimize neuroplasticity. Delays in diagnosis can be harmful to parent’s and caregiver’s well-being. A diagnosis allows parents to receive psychological support and resources. Patients with known risk factors for cerebral palsy should receive a referral for further diagnostic testing, including neuroimaging and standardized developmental assessments.[10] Following the best treatment, a significant reduction in the lifespan is usually seen in most individuals with cerebral palsy.[20]