Continuing Education Activity
Atrial flutter, a supraventricular arrhythmia, is one of the most common rhythm disturbances of the heart. It is characterized by a fast atrial rate with a fixed or variable ventricular rate. There are several atrial contractions to one ventricular contraction and symptoms include fatigue, palpitations, and syncope. This activity outlines the evaluation and treatment of atrial flutter and highlights the role of the interprofessional team in managing patients with this condition.
Objectives:
- Identify the role of the re-entrant mechanism in the etiology of atrial flutter.
- Describe the different pathophysiologies of typical and atypical atrial flutter.
- Summarize the use of electrocardiogram, echocardiogram and laboratory tests in the evaluation of atrial flutter.
- Review the importance of improving care coordination among the interprofessional team members to decrease the risk of embolic stroke and improve outcomes for patients affected by atrial flutter.
Introduction
Supraventricular arrhythmias are a diverse group of atrial arrhythmias. Atrial fibrillation and atrial flutter are the most common of these atrial arrhythmias, and the other less common supraventricular arrhythmias are atrial tachycardias, atrioventricular reentrant tachycardia, atrioventricular nodal tachycardia, and others.
In this review will summarize the management of atrial flutter.
Atrial flutter is one of the most common arrhythmias and is characterized by an abnormal cardiac rhythm that is fast with an atrial rate of 300beats/min and a ventricular rate that can be fixed or be variable that can cause palpitations, fatigue, syncope, and embolic phenomenon.[1]
Atrial flutter is a macro-reentrant tachycardia and depending on the site of origin can be typical or atypical atrial flutter.[1] Electrocardiographic findings of atrial flutter are flutter waves without an isoelectric line in between QRS complex. Electrical axis of the flutter waves can help to determine the origin of the atrial flutter.
Typical or cavotricuspid isthmus (CTI) dependent is the most common type of atrial flutter; this rhythm originates in the right atrium at the level of the tricuspid valve annulus. Typical atrial flutter is seen in the electrocardiogram as continuous negative modulation in inferior leads (II, III, and AVF) and flat atrial deflections in leads I and aVL; this is due to the way of propagation and activation of the macro-reentrant circuit as will be described in the pathophysiology section.
The atypical atrial flutter is independent of the CTI, and the origin of the arrhythmia can be in the right atrium or the left atrium.
Less commonly, atrial activation can be in a clockwise fashion, and thus electrocardiographic appearance is different, one is unable to differentiate it easily from not isthmus-dependent atrial flutter.
Etiology
The etiology behind atrial flutter is the presence of a re-entry mechanism for initiation of the tachycardia.
To have this electrical circuit, one must have the following elements[2]:
1. Areas with fast and slow velocities of conduction
2. Different refractory periods
3. A functional core where the circuit exists
These elements are present in a typical atrial flutter in the CTI. The initiation of atrial flutter is due to an ectopic beat that depolarizes one segment of the pathway of the circuits that become refractory and starts the tachycardia from a no-refractory segment.[3]
Epidemiology
Atrial flutter is the second most common cardiac arrhythmia after atrial fibrillation.[4]
It is commonly associated with atrial fibrillation, but the incidence and the prevalence of the atrial flutter are less known when compared with atrial fibrillation.[4]
Atrial flutter in common in patients with underlying diseases such as chronic obstructive pulmonary disease, pulmonary hypertension, and heart failure.
Isolated atrial flutter in the absence of abnormal heart anatomy is rare and usually is present when atrial size abnormalities have developed.[5]
Atrial flutter is more frequent in males than in females. Aging is a significant risk factor as other associated disorders in patients with atrial fibrillation include systemic hypertension, diabetes mellitus, and history of alcohol abuse.
Older age is associated with an increased risk of atrial fibrillation and atrial flutter.
Pathophysiology
Typical Atrial Flutter
Typical atrial flutter is the most common type of atrial flutter and is a macroreentrant atrial tachycardia that uses the CTI as an essential part of the circuit. The Todaro tendon, crista terminalis, the inferior vena cava, the tricuspid valve annulus, and the coronary sinus os delineate the circuit. These structures are essential to provide the pathway length for the flutter system.
The CTI provides the slow conduction pathway, and it presents in the lateral aspect of the younger patient and the medial aspect in the older patients. The mechanism of slow conduction is not well understood but might be related to anisotropic fiber orientation. With aging and atrial dilation, occurs fibrosis of the atrial tissue and produce non-uniform anisotropic conduction through the CTI.
The crista terminalis is a functional barrier that induces a transverse conduction block, steep slope, and arborization that allows the circuit to exist.
The mechanism of arrhythmia is a macro-reentry activation of the right atrium from the interatrial septum and along the crista terminalis with passive activation of the left atrium via the coronary sinus muscular connection.[6][7]
As this cycle occurs in the atrium, conduction is determined by the atrioventricular node mechanism to conduct the atrial impulse. Commonly the atrioventricular conduction will be 2 to 1 with an atrial rate of 300 beats per minute with a ventricular rate of 150 beats per minute, but this can be variable depending on the underlying parasympathetic stimulus or refractoriness of the atrioventricular node.
The absence of an isoelectric line between P waves or QRS complexes is due to the constant cycling of the circuit or atrial activation.
The reason behind the existence of the circuit might relate to the nature of the anatomical structures that are circumscribing the circuit. The crista terminalis thickness might have the capacity to block conduction, as well as the low voltage of the CTI, can be signs of arrhythmogenesis and poor conduction in the right atrium.
Atypical Atrial Flutter
Atypical atrial flutter or other macroreentrant atrial tachycardia has a circuit configuration different from the typical right atrial flutter circuit. Electrophysiologic studies and intracardiac mapping are the only means to determine the exact mechanism or area generating the atrial flutter. Different from typical atrial flutter, the presence of atypical atrial flutter is related to structural heart diseases as prior cardiac surgery or ablation procedures.[8]
When the atrial flutter is determined to come from the right atrium but not associated with the CTI system, the circuit can be in the superior vena cava and part of the terminal crest. When prior surgery or intervention occurred, the presence of scar can often become arrhythmogenic, and the center of the circuit and the onset of the arrhythmia mostly occur after several years of the procedure, likely secondary to remodeling.[9]
In patients without prior cardiac intervention, the atrial flutter circuit can be low voltage areas like the lateral right atrium,[10] this might be secondary to fibrosis due to chronic atrial high pressures, or cardiomyopathy that can produce fibrosis of the myocardium and creating low voltage areas that allow atrial flutter to occur.[10]
Left atrial flutter can be associated with surgical atriotomy scars or areas of prior ablations, combined with areas of low voltage.[11]
Electrophysiologic studies and mapping of the right and left atrium are necessary to determine the specific location and mechanism of the arrhythmia to guide the ablation. In the presence of an intra-atrial septal macro-reentrant system, the success rate is low when compared with the free wall atrial tachycardias.[12]
History and Physical
Patients with atrial flutter can be asymptomatic or present with symptoms as palpitations, lightheadedness, fatigue, and shortness of breath especially in the presence of rapid ventricular conduction.
Decreased exercise tolerance is another symptom that can be present during patient evaluation.
During the rapid ventricular rate, hypotension, syncope and near syncope can occur in susceptible patients with high ventricular rates.
Some patients remain asymptomatic until they develop acutely decompensated heart failure, tachycardia-induced cardiomyopathy, and embolic stroke.
The physical exam in patients with atrial flutter will show regular or irregularly regular peripheral pulse (due to variable conduction from the atrioventricular node), jugular venous distension, respiratory sounds with crackles in lung fields, tachycardia, abdominal distention, and lower extremities edema when congestion occurs.
Evaluation
Initial evaluation of the underlying rhythm is necessary, and determination of possible etiology or trigger is crucial.
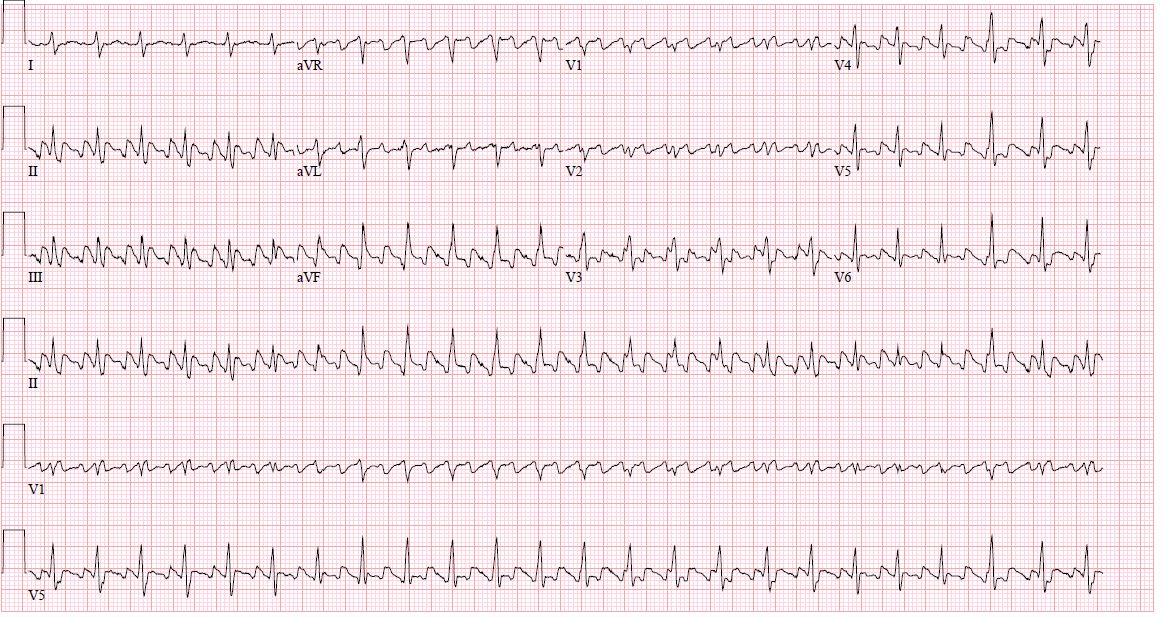
Electrocardiogram:
Electrocardiogram generally shows flutter waves with the absence of an isoelectric line between QRS complexes, with an atrial wave around 300 beats per minute with ventricular conduction that can be 2 to 1, 3 to 1 or 4 to 1 or with variable conduction due to Wenckebach phenomenon.
In inferior leads, typical flutter waves resemble a picket fence or sawtooth because the P waves are negative due to the direction of the vector.
Typical atrial flutter with counterclockwise activation will show inferior leads with negative flutter waves with low amplitude in lead I and upright flutter wave in aVL.[9]
Echocardiogram
Echocardiography for the evaluation of atrial flutter bases its value on the determination of underlying structural heart disease. Presence of dilated atrial chambers is a sign of chronicity and also fibrosis of the atrium that could make the circuit persist and more challenging to control.[13]
Assessment of left ventricular ejection fraction can be the cause or consequence of underlying atrial flutter because the persistence of tachycardia can generate tachycardia induced-cardiomyopathy or the cardiomyopathy and volume status can trigger the atrial flutter.
Evaluation for atrial or ventricular thrombus is also important, especially when desiring cardioversion to sinus rhythm. A transesophageal echocardiogram is the modality of choice because it can visualize the atrial appendage where atrial thrombus is more frequently present.[14]
Laboratory evaluation
An initial determination of atrial flutter triggers is necessary. Laboratory evaluation of electrolytes disturbance, abnormal thyroid function, infection, anemia, hypoxia. Correction of these abnormalities can improve symptoms and decrease the threshold of development of atrial flutter and rapid ventricular response.
Pulmonary function test might be necessary for this set of patients; there is a correlation between lung disease and presence the atrial arrhythmias including atrial flutter. Management of the underlying lung condition can improve the control of the atrial flutter.
Treatment / Management
Treatment management should focus on the following aspects:
- Rhythm control
- Rate control
- Anticoagulation due to embolization risk
1. Rhythm control
Maintenance of the sinus rhythm or conversion of the sinus rhythm is essential.
Persistence of atrial flutter, can cause chronic remodeling of the atrium and make it more difficult to manage the rate and the conversion or maintenance of sinus rhythm.
There are different ways to achieve sinus rhythm: with electrical cardioversion, pharmacological cardioversion and ultimately with catheter ablation.
The rhythm strategy divides into acute and long term management.
In the acute setting, in patients with atrial flutter who are hemodynamically unstable, synchronized cardioversion is indicated for the conversion of sinus rhythm to stabilize the patient.[15]
In stable patients, pharmacological cardioversion is achievable with different antiarrhythmics. Antiarrhythmic drugs like amiodarone, class IA (procainamide, quinidine, and disopyramide) and IC drugs (flecainide and propafenone), calcium channel blockers (verapamil, diltiazem), and beta-blockers (metoprolol, carvedilol, esmolol) are some of the choices for pharmacological cardioversion.[16]
Despite the multiple pharmacological options the control and conversion of atrial flutter to sinus rhythm is difficult. The mechanisms by which antiarrhythmic drugs maintain sinus rhythm is by prevention of premature beats that usually start the activation of the tachycardia circuit by a reentrant or an ectopic beat.
In the presence of a new diagnosis of atrial flutter, one should start the patient on anticoagulation. In the absence of intracardiac thrombus, electrical cardioversion in stable patients can be considered, especially to prevent persistence of the arrhythmia and further fibrosis that will perpetuate the presence of atrial flutter and more difficult to control or to convert to sinus rhythm.[17]
In patients who have a contraindication to these drugs or do not tolerate them may consider catheter ablation of the atrial flutter circuit.[18]
Radiofrequency catheter ablation of the CTI is the standard treatment for typical atrial flutter with a success rate of 95% with few complications post-procedure.[19] The procedure consists placement of intracardiac catheters into the coronary sinus, the atrium, and an ablation catheter. The anatomical target for the CTI is found through a mapping and entrapment technique. After this, the linear lesion is made by the ablation catheter with the use of radiofrequency energy. At the end of the ablation when the line is complete, verification bidirectional conduction block and absence of atrial flutter is done to confirm ablation is complete. In the rare cases of medication and ablation failure, atrioventricular nodal ablation with the placement of a pacemaker might be indicated to prevent atrial to ventricular conduction of rapid atrial flutter.
2. Rate control
Rate control is achievable with the use of atrioventricular nodal agents as calcium channel blockers (first line) or beta blockers.[20] Digoxin is another option for rate control but needs to be used carefully due to its side effects and toxicity.
Combination of these agents is an option.
Adequate control of the atrial flutter through AV nodal agents is difficult because atrial flutter continuously fires at the same rate to the AV node.
The heart rate goal should be below 110 beats per minute.[21] This was determined after the RACE II trial (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient versus Strict Rate Control II). This study compared strict heart rate control of <80 bpm versus <110bpm, this study showed that very stringed heart rate control is not necessary and more lenient control reduces polypharmacy and fewer side effects and less outpatient visits. This study was in patients with atrial flutter but can extrapolate to the rate control of atrial flutter.
In patients who are intolerant of medications or have significant bradycardia due to rate control measures, a catheter ablation is an option.
3. Anticoagulation due to the risk of embolic events:
Patients with atrial flutter have a similar risk of strokes as those with atrial fibrillation.[22]
Use of a scoring system to determine the annual risk of stroke must be used such as the CHADS2-Vasc.[23] This scoring system helps to risk stratify the patient according to their risk of developing embolic strokes due to atrial flutter or atrial fibrillation. The presence of congestive heart failure, systemic hypertension, diabetes, female sex, age between 65 to 74 years and a history of the peripheral vascular disease score as one point for each comorbidity that is present.
Age more than 75 years old and a history of stroke are two additional points each.
The presence of one point can give the patient an annual risk of 1.3% of embolic stroke per year when two points the risk is 2.2%. In patients with two or more points, the use of anticoagulation is a strong recommendation. In patients with one point, one may use either aspirin or full anticoagulation.
Differential Diagnosis
- Atrial fibrillation: mostly irregular, no evidence of organized atrial activity in the electrocardiogram, absence of P waves
- Multifocal atrial tachycardia: multiple P waves morphology due to the presence of several atrial pacers
- Atrial tachycardia with variable conduction: the isoelectric line between QRS complexes
Prognosis
Prognosis of patients with typical atrial flutter undergoing catheter ablation is good with a recurrence rate of less than 5%.
Persistence of atrial flutter can generate tachycardia induce cardiomyopathy that is hard to control causing multiple hospitalizations due to decompensation.
Complications
The most common complication of atrial flutter is the increased risk of embolic stroke and disability related to this event.
Hemodynamically instability is also possible especially in patients with a rapid ventricular response.
Chronicity and poor control of atrial flutter can generate tachycardia induce cardiomyopathy and also can produce hard to control heart failure.
The complications secondary to the use of antiarrhythmic drugs are related to the type of drug and underlying mechanism of the drug.
Atrial flutter ablation complications also depend on the side of the origin of the atrial flutter. Right-sided atrial flutter is related to fewer complication rates than left-sided atrial flutter ablation, and this is due to the need for creating a transeptal communication during the procedure to reach the left atrium foci of arrhythmia and perform the ablation. The transseptal puncture produces transient communication between the left and right chambers of the heart. There is also increasing the risk of embolic strokes with left-sided atrial flutter ablation when compared with right side procedures.
Enhancing Healthcare Team Outcomes
There is no cure for atrial flutter and the disorder is known to be associated with a high risk of a stroke and other embolic phenomena; this the management of atrial flutter is best done by an interprofessional team including physicians, specialists, pharmacist, and a cardiac specialty nurse is recommended. While the cardiologist may initiate the initial treatment, the majority of patients are followed by the primary provider or nurse practitioner. These patients need life-long follow up because there is a risk of an embolic stroke. If the disorder receives inadequate therapy, it leads to a poor quality of life.
The primary care providers, pharmacists, and nurse practitioners should enforce medication compliance. Those patients on warfarin should have a dietary consult because a sudden change in the consumption of green leafy vegetables can affect the INR. The hematology nurse and pharmacist should monitor the INR but the dose adjustment of warfarin should be done by the clinician.
At each clinic visit, an ECG should be performed and the patient examined for any embolic phenomenon. To improve outcomes, open communication between members of the interprofessional team is essential.
Outcomes
Despite advances in treatment, recurrence and multiple admission to the hospital are very common.

![A Flutter, [SATA]](/pictures/getimagecontent//6481)