Introduction
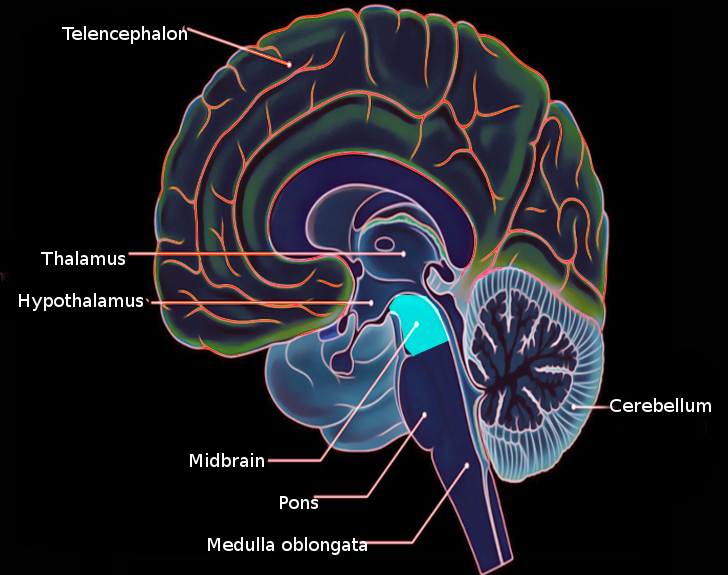
The brainstem, including the midbrain, the pons, and the medulla, is composed of several nerves, pathways, reflex centers, and nuclei (see Image. Midbrain Anatomy). The midbrain is the smallest portion of the brainstem (about 1.5 cm) and its most cranial structure. It is in the brainstem between the pons caudally (mesencephalic-pons groove) and the diencephalon, which includes the thalamus, the hypothalamus, the epithalamus, and the subthalamus.[1]
The midbrain mediates the reflexes of miosis and mydriasis and plays an essential role in sensory and motor control pathways. It contains, for instance, several extrapyramidal structures that are important for movement regulation. Furthermore, it is indispensable for the passage of impulses from the nervous system between the spinal cord and brain.[2] Throughout the brainstem, there are ascending pathways and descending systems that carry either motor or sensory information. Schematically, sensory information travels by the ascending pathways to be processed, and then descending pathways create the motor response to the sensory input. In functional terms, however, the matter is much more complicated. There are, for example, descending pathways of sensory modulation, of considerable importance in nociception mechanisms.[3] Again, the functions of the midbrain also concern the mechanisms of consciousness and sleep. The role of some mesencephalic areas (especially of the midbrain tegmentum and of the reticular formation) in the awakening phases (emergence) from general anesthesia has also been investigated.[4]
Structure and Function
Cerebral Aqueduct of Sylvius
The midbrain connects at its posterior aspect to the cerebellum via the superior cerebellar peduncles. On its anterior surface, it is recognizable the crus cerebri, which carry fibers such as motor cortical spinal fibers and fibers from the nuclei found within the pons. The midbrain contains the centrally located cerebral aqueduct. Through the aqueduct, the cerebrospinal fluid (CSF) passes from the third ventricle into the fourth ventricle.
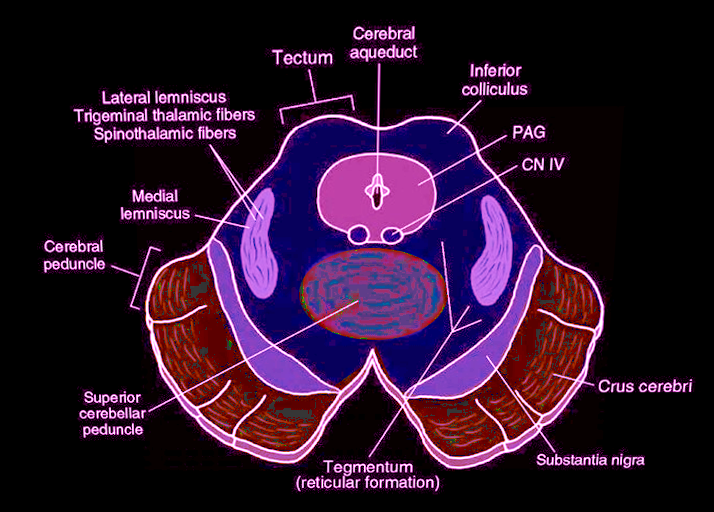
In practical terms, the midbrain a distinguishable into tegmentum, the ventral part, and tectum, the dorsal part. The former includes different structures, such as the cranial part of the reticular formation, cranial nerve nuclei (which control eye movements,) the periaqueductal gray (PAG) matter, the red nucleus, the substantia nigra, and the ventral tegmental area (VTA). On the other hand, the midbrain tectum consists of the corpora quadrigemina and is responsible for auditory and visual reflexes. See Image. Midbrain Cross-Sectional Anatomy.
Midbrain Tegmentum
The midbrain reticular formation
This term indicates several phylogenetically old nuclei located throughout the brainstem. These nuclei form numerous networks related to visceral (e.g., cardiovascular control) and movement functions and implicitly play a role in the state of consciousness and the waking and sleeping rhythms. This system is also involved in the mechanism of pain modulation.[5] The neurons that constitute the reticular substance is definable as isodendritic as they have thick dendrites of more or less equal length. These networks lie in the core of the brainstem and project to the whole brain. Functionally, it comprises ascending pathways until the cortex (extrathalamic control modulatory system or ascending reticular activating system: ARAS) and descending pathways to the spinal cord through the reticulospinal pathways. The reticular formation divides into three columns. These columns form the serotoninergic raphe nuclei (median) implicated in the mood regulation and pain modulation; gigantocellular reticular nuclei (medial zone) essential for motor coordination; and parvocellular reticular nuclei (lateral zone), which is involved in the respiratory regulation (exhalation). The raphe nuclei complete the locus coeruleus, which acts as an activator of the cortex related to alert situations, and attention.
Periaqueductal gray (PAG)
Around the aqueduct, there is a grey matter called the PAG matter, which is the primary control center for descending pain modulation and consists of neurons containing neurotransmitters such as enkephalin, dynorphin, and serotonin. Because the instinct to avoid painful situations is essential to guide behavior, it plays a pivotal role in autonomic function, motivated behavior, and behavioral responses to threatening stimuli. Thus, PAG is the region where the discrepancy between the amount of expected pain and actually perceived becomes elaborated. In practice, the PAG matter receives and elaborates information on the pain perceived directly from the spinal cord, while those on the expected pain sensation come from other brain regions, such as the ventromedial prefrontal cortex. This arrangement is a survival strategy that allows inhibition of behaviors considered dangerous.
Cranial nerves nuclei
The nuclei of two pairs of cranial nerves are at the ventral side of the PAG. These nuclei are the oculomotor nuclei which through the oculomotor nerve (III) is responsible for the control of the pupil and is involved in the control of most eye movements, and trochlear nuclei from which emerges the trochlear nerve (IV), which is the only pair of nerve to emerge from the midbrain in a dorsal position. It is a motor nerve that innervates the superior oblique muscle of the eye. The location of the Edinger-Westphal nucleus (a parasympathetic pre-ganglionic nucleus that innervates the iris sphincter muscle and the ciliary muscle) is between the oculomotor nucleus and the Sylvius aqueduct.
Ascending and descending pathways
One major ascending pathway that crosses the midbrain is the spinothalamic tract, which carries pain and temperature sensation and passes through the midbrain on its way to the thalamus. The major descending motor pathway is the corticospinal tract, which descends through the crus cerebri on its way to the anterior pons, and eventually to the medullary pyramids where they cross. Another descending tract is the rubrospinal tract, which originates within the midbrain in an area known as the red nucleus and inhibits other motor pathways.[6][7]
The midbrain also contains many nuclei such as the red nucleus and the substantia nigra, which play a role in multiple pathways and disease processes.[2] These structures are observed in cross-section and have a different color according to the pigment they contain.
Red nucleus[8]
The red nucleus (so-called because of the reddish color due to the iron content) divides into a caudal (magnocellular) portion and a rostral part (parvicellular). The magnocellular portion originates the rubrospinal bundle, which constitutes the tegmental decussation and descends along the brainstem and spinal cord, ending in the laminae V, VI, and VII of the spinal cord. The rubrospinal tract seems to be useful for guiding the movements of the lower limbs and for restraining those of the upper limbs. For example, the movement that takes place when we move our arms when we walk depends on this modulation. They are automatic movements that we also do to help our balance. The red nucleus receives afferents from the telencephalic cortex (in particular from the areas responsible for motor control) in addition to the afference coming from the contralateral cerebellar nucleus interpositus. The parvicellular component, more important in humans, is involved in the motor control exercised by the cerebellum. It receives afferents from the dentate nucleus of the contralateral cerebellum and sends efferent fibers to the inferior olive nucleus (ION) following the path of the central tegmental tract. The neurons of the ION project to the contralateral cerebellum through the climbing fibers. Therefore, a cerebellum-red nucleus-ION circuit forms. Red nucleus lesions usually cause a disorder characterized for the most part by a contralateral tremor of the lesion and deficits in motor coordination.
Sommering's substantia nigra
The Sommering's substantia nigra is a lamellar structure that, like the red nucleus, is located in an intermediate position between the midbrain and the diencephalon and, although its rostral extremity goes deep into the subthalamus, it is conventionally assigned to the midbrain.
The substantia nigra, whose name is due to the presence in the neurons of high amounts of melanin pigment, contains dopaminergic neurons which either inhibit or increase the release of dopamine to control movements in association with the basal ganglia. The functionality of Sommering's substantia nigra is distinguished by its two parts, pars reticulata and pars compacta. The pars reticulata contains GABAergic neurons, and together with the globus pallidus (paleostriatum), it constitutes one of the two output nuclei of the basal nuclei. Pars reticulata and paleostriatum send inhibitory impulses on thalamic motor activity. This action is under the regulation of the inhibitory activity of the striatum. The afferent fibers to the reticulated substantia nigra come from the caudate nucleus and from the putamen (which together make up the striatum). In contrast, it sends efferents to the anterior and lateral thalamic ventral nuclei (via nigro-thalamic), and other fibers go to the superior colliculi. The pars compacta of the substantia nigra projects above all to the basal striatum through the nigrostriatal pathway. This route constitutes the so-called diffuse projection dopaminergic system, which has a crucial modulatory role. This system contains D1 and D2 receptors. The former are exciters on the neurons involved in the direct pathway, while the latter (D2) are inhibitors on the neurons involved in the indirect pathway and on the cholinergic interneurons of the activating system of the nuclei of the base. In this way, the nigrostriatal pathway can excite the neurons of the direct pathway and inhibit those of the indirect pathway, with a global meaning of movement facilitation. The afferent fibers to the pars compacta come from the striated body and the globus pallidus. Other projections come from the cortex. The efferent fibers get directed to the striatum.
Ventral tegmental area
This term, also called the VTA of Tsai, indicates a group of neurons found close to the midline on the midbrain floor. This neuronal aggregate is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine-based circuits. The distinctive morphofunctional characteristics of these cells are manifold. For example, research has shown that VTA has a vast network of GABAergic neurons that interconnect via gap junctions, which feature a very fast, although poorly controlled, conduction. This structure, thus, is the subject of numerous studies as it is involved in the mechanisms of drug and the natural reward circuitry of the brain.[9] Consequently, the VTA represents a key region for processes of reward cognition such as motivational salience, associative learning, and positively-valenced emotions. It also has implications in the mechanism of the orgasm. Of note, the VTA project to numerous areas of the brain involved in the mechanisms of consciousness and sleep, such as the prefrontal cortex and the occipital cortex.[10]
Midbrain Tectum
Corpora quadrigemina
On the posterior surface of the midbrain, there is a structure called the corpora quadrigemina. It is composed of superior and inferior colliculi. Superior colliculi are two multilayers structures located below the thalamus and around the pineal gland and involved in processing visual information. Inferior colliculi are smaller than superior and divided in a central nucleus and dorsal and external cortex. Functionally inferior colliculi are involved in the processing of auditory information. The raphe nuclei which contain serotonergic neurons are at the ventral side of the PAG at the level of the inferior colliculus.
Embryology
The entirety of the central nervous system (CNS) derives from neuroectoderm. Ectoderm is one of the three initial germinal layers of the CNS, along with mesoderm and endoderm. This neuroectoderm eventually becomes the neural tube and neural crest, from which form the brain and nerves that make up the CNS. During the formation of the brain itself, the neural tube induces the formation of three vesicles; the prosencephalon, the mesencephalon, and the rhombencephalon. After this stage, the three vesicles differentiate into five vesicles, with the mesencephalon becoming the midbrain and the aqueduct.[11]
Blood Supply and Lymphatics
The internal carotid and vertebral arteries form the network of blood vessels that supply blood to the brainstem, including the midbrain. Anteriorly, the midbrain receives supply from branches of the basilar artery, laterally by branches of the posterior cerebral artery (PCA), including the posterior choroidal arteries and quadrigeminal artery, and posteriorly by quadrigeminal arteries and superior cerebellar arteries (SCA).[7]
There is no classical lymphatic drainage system within the central nervous system (CNS). When trying to identify exit routes for immune cells after immunohistochemistry staining, they travel within the meninges near the dural sinuses. These sinuses are what drain blood from the brain into the internal jugular veins.[12] The CNS lymphatic system is not fully understood but is believed to be a means of transport of immune cells, as stated, and cerebrospinal fluid (CSF) and interstitial fluid.[7]
Nerves
Two vital cranial nerves that are present within the midbrain are cranial nerve III, the oculomotor nerve, and cranial nerve IV, the trochlear nerve. The oculomotor nerve has a portion that lies between the cerebral peduncles located in the ventral midbrain while the trochlear nerve comes out of the midbrain dorsally.[2] The oculomotor nerve is responsible for the motions of several muscles that serve to raise the eyelid and move the eye upward and medially. It is also responsible for the constriction of the pupil through the sphincter pupillae muscle and for accommodation through the ciliary muscles.[13] The trochlear nerve is responsible for the innervation of the superior oblique muscle that moves the eye downward. Disruptions to these nerves along their pathway lead to distinct syndromes.[14]
Physiologic Variants
There are a significant number of recognized malformations of the brainstem, which can each lead to specific presentations. There are, however, few disorders known that affect mostly the midbrain. One condition caused by midbrain dysplasia results in many symptoms in affected people, including microcephaly, intellectual disability, and seizures.[15] In some patients with dysfunction of multiple organ systems, a shortened midbrain and elongated pons were found. This malformation was due to the malfunction of development along the anterior-posterior axis. Another disorder caused by the opposite problem, an elongation of the midbrain, results in a secondary hypoplastic cerebellum.[16]
Surgical Considerations
When performing surgery on this area of the brain, it is especially important to be aware of its internal anatomy. Many parts of the midbrain are not distinct. When focusing on one structure, an adjacent structure can easily be affected; this is mainly why recent techniques have focused on improving microsurgery, imaging, and knowledge of the internal anatomy of both the intended area and the surrounding areas.[1]
Clinical Significance
Since the oculomotor nerve and the trochlear nerve run through the midbrain, a lesion at the midbrain could cause a palsy of either nerve. An oculomotor nerve palsy would present with the eye positioned in a downward and outward direction because the eye cannot look inward or upward. It would also present with a dilated pupil, droopy eyelid, diplopia, and no accommodation.[13] A trochlear nerve palsy would present with a deviation of the eye upwards, blurry vision, diplopia, and tilt of the head towards the unaffected side to compensate for visual changes.[14]
When presenting with midbrain pathology symptoms, it is possible to determine which part of the midbrain has been affected depending on what symptoms present, even though overlapping symptoms may be present. The syndromes got their names according to the location of the lesion within the midbrain. Parinaud syndrome, or dorsal midbrain syndrome, is one example. The syndrome is inducible by ischemic lesions, neoplasms of the corpora quadrigemina, pinealomas, hydrocephalus, cysticercosis, multiple sclerosis, and arteriovenous malformations. Its distinguishing symptom is a decreased or absent gaze upwards.[17] Weber syndrome, or ventromedial midbrain syndrome, is caused by mesencephalic ischemia due to the occlusion of the paramedian branches of the PCA or involving a basilar perforating artery. Clinically, it presents with oculomotor nerve palsy on the same side as the lesion and weakness on the opposite side of the lesion.[2] When the red nucleus is interrupted, then the rubrospinal tract is interrupted as well, which leads to an inability of the tract to carry motor information and results in extensor posturing.[6]
Apart from lesional syndromes (for example, on a vascular basis or due to cancers), a series of pathologies of considerable medical interest concern the midbrain. The essential pathology associated with a mesencephalic alteration is Parkinson disease (PD). It is a neurodegenerative disorder with substantial morbidity and mortality and featuring a progressive disabling motor, cognitive impairment, and non-motor symptoms (NMS) such as mood disorders, sleep alterations, and pain.[18] Interestingly, following Alzheimer disease, PD is the most common neurodegenerative disorder with a prevalence of approximately 1% of the population above 60 years.[19] The main pathological characteristic of PD is the neuronal degeneration involving the cells of the substantia nigra and, more specifically, the ventral part of the pars compacta, causing the death of the cells over time up to 70% of their total. In PD, the histopathology of the substantia nigra and the different brain regions involved shows neuronal loss and Lewy bodies (a key pathological element in PD) in many of the remaining nerve cells. Neuronal loss accompanies astrocyte death and microglial activation. The degenerative process becomes progressively more extensive as all the circuits that connect the brain areas to the basal ganglia (i.e., motor circuit, oculomotor circuit, associative circuit, limbic circuit, and orbitofrontal circuit) can be influenced, and this explains the different clinical expression of the pathology and, in turn, the presence of NMS.
Another degenerative condition is the Steele-Richardson-Olszewski syndrome or Progressive supranuclear palsy (PSP), in which the neurodegeneration involves atrophy in the midbrain (substantia nigra) and other brain structures, including the subthalamic nucleus and the globus pallidus.[20]
The reticular substance is also involved in the genesis of pathological conditions. Researchers have identified ARAS functional alterations in various sleep disorders, such as narcolepsy. Degeneration in the ARAS is also present in the early stages of PD.[21]