Continuing Education Activity
Cerebellar infarcts, encompassing both infarcts and hemorrhagic events, constitute a subset of cerebrovascular events that specifically impact the posterior cranial fossa, targeting the cerebellum. These strokes impair perfusion, leading to diminished oxygen delivery and significant motor and balance control deficits. Hemorrhagic events further worsen these deficits through direct tissue damage from bleeding. Despite their relatively low frequency, cerebellar strokes carry notable morbidity and mortality, attributed to the challenges arising from their occasionally subtle initial presentation and the adverse effects of reactive swelling in the posterior fossa. Accounting for 1% to 4% of all brain strokes, cerebellar infarcts demand attention due to their unique challenges in diagnosis and management.
This course explores the mechanisms leading to impaired perfusion and the resulting deficits in motor and balance control. The unique challenges cerebellar strokes pose and the importance of an interprofessional approach in their management are emphasized. Participants acquire practical knowledge for the comprehensive workup of cerebellar infarcts, highlighting the collaborative role of healthcare professionals in optimizing patient outcomes.
Objectives:
Identify the early symptoms associated with cerebellar infarcts, including severe headache, altered mental status, vomiting, and drowsiness, to enable prompt recognition and intervention.
Screen patients with suspected cerebellar infarcts for progressive edema and signs of herniation, emphasizing the importance of continuous monitoring, especially within the first 24 hours.
Assess the need for advanced interventions, such as extraventricular drains, ventriculostomy, or decompressive sub-occipital craniotomy, in cases of large strokes with elevated intracranial pressure.
Coordinate with healthcare professionals to enhance screening practices, facilitate early intervention, and engage in shared decision-making, ultimately improving the quality of life for individuals affected by cerebellar strokes.
Introduction
A cerebellar infarct, or cerebellar stroke, is a cerebrovascular event involving the posterior cranial fossa, specifically targeting the cerebellum. Reduced perfusion impairs oxygen delivery, leading to motor and balance control deficits. In cases of hemorrhagic events, bleeding can directly damage tissue, exacerbating these deficits. Despite constituting a small fraction of all strokes, cerebellar strokes contribute significantly to morbidity and mortality. This is due to their often subtle initial symptoms and the adverse effects of reactive swelling in the posterior fossa. Cerebellar strokes account for 1% to 4% of all brain strokes.[1][2]
Etiology
Like all strokes, cerebellar infarcts can be broadly categorized into ischemic and hemorrhagic events. Ischemic strokes result from arterial obstructions that directly impair blood and oxygen delivery. These obstructions can originate either from migration from the heart or occur directly within the vasculature. The thrombotic phenomenon can affect large and small vessels, and thromboembolic events can travel from larger to smaller vessels. In cerebellar strokes, these obstructions may be caused by atherosclerosis or other vasculopathies, including arterial dissections.[3][4]
Emboli originating from the heart often occur due to pump failure or irregular heart rhythms, disrupting smooth blood transit through the heart, as seen in conditions like atrial fibrillation or atrial flutter. Additionally, emboli can disseminate from the venous circulation in the presence of a right-to-left shunt, such as a patent foramen ovale. In cases of cerebral venous thrombosis, obstructed venous outflow can lead to either infarction or hemorrhage.
Unlike ischemic strokes, hemorrhagic strokes typically result from arterial bleeding that directly damages brain tissue or obstructs vascular flow due to elevated local pressure. These incidents often occur spontaneously, especially in patients with long-standing hypertension or those taking anticoagulants or antiplatelet agents. Secondary hemorrhagic conversion can also arise from ischemic infarcts, as well as from damaged tissue due to a tumor or trauma. Additionally, subdural hematomas, which usually originate from tears in bridging veins between the dura and arachnoid mater, can lead to focal neurologic deficits.
Epidemiology
Cerebellar infarcts make up only about 2% of all strokes, yet they contribute significantly to the resulting morbidity and mortality. A study involving nearly 2000 consecutive stroke patients revealed that cerebellar strokes had an alarming mortality rate of 23%, almost twice that of more common cerebral strokes (12.5%). Brainstem strokes fell in between, with a mortality rate of 17%.[5]
Pathophysiology
Neurological deficits in cerebellar infarcts are primarily determined by the physiologic function of the involved vascular territories (see Image. Cerebellar Arteries and Distribution). Obstruction of the posterior inferior cerebellar artery (PICA), the most frequent site for cerebellar infarcts, results in a headache and, less commonly, symptoms like vomiting, vertigo, horizontal ipsilateral nystagmus, and truncal ataxia. Infarction in the territory supplied by the anterior inferior cerebellar artery (AICA) often leads to dysmetria, Horner syndrome, unilateral hearing loss, and ipsilateral facial paralysis or anesthesia with contralateral hemibody sensory loss of pain and temperature. Obstruction of the superior cerebellar artery (SCA), located more rostrally, tends to produce more ataxia, dysarthria, and nystagmus, with less frequent vertigo, headache, and vomiting occurrences.[6] However, presentations can often be atypical or overlap, especially in the case of hemorrhagic infarcts.
Reactive cerebral edema surrounding the territory of an initial infarct can pose significant challenges, particularly in the context of cerebellar strokes. The cerebellum is situated within the confined space of the posterior cranial fossa, enclosed by the tentorium cerebelli above, the occipital bone behind and beside it, the foramen magnum below, and the fourth ventricle and brainstem in front. Swelling can lead to upward transtentorial herniation of the cerebellar vermis, a mechanism the brain employs to alleviate the increasing intracranial pressure.
Alternatively, edema can anteriorly obstruct the fourth ventricle and aqueducts, causing direct brainstem compression. As intracranial pressure increases, starting from the choroid plexus superiorly, where cerebrospinal fluid is synthesized and transmitted downwards through the brain, obstruction of the fourth ventricle can create significant downward pressure, leading to a catastrophic herniation of the cerebellar tonsils into the foramen magnum.
History and Physical
The presenting symptoms of a cerebellar infarction are frequently nonspecific and can overlap with other neurological, cardiovascular, gastrointestinal, and systemic conditions.[7] It is crucial to focus on thoracic, abdominal, and systemic complaints and findings, as this attention can help rule out other potentially life-threatening conditions such as trauma, aortic dissection, acute coronary syndrome, pulmonary embolism, hypovolemia, or sepsis.
Cerebellar infarctions are often misdiagnosed as vestibular neuronitis, migraine, syncope (potentially due to orthostasis or arrhythmia), hypertensive emergency, renal failure, hypoglycemia, or ethanol or drug intoxication. Like other types of strokes, paying attention to historical features is crucial for risk stratification and treatment planning.
The timing of symptom onset and progression, coupled with provoking or alleviating factors, can be invaluable in diagnosing cerebellar strokes and distinguishing them from other conditions. Symptoms appearing within seconds to minutes are typically associated with benign paroxysmal positional vertigo (BPPV), vasovagal syncope, or arrhythmia. Signs emerging over minutes to hours are often due to hypoglycemia, migraine, or psychological causes. Conversely, symptoms developing over hours to days are attributable to labyrinthitis or medication side effects but also raise suspicion for a cerebellar infarct.
Prolonged symptoms may evoke suspicions of an expanding mass in the posterior fossa. Symptom triggers provide diagnostic cues, like loud noises suggesting inner ear issues or relief upon eye closure indicating an ophthalmologic origin. Changes in body posture implicating a cardiovascular connection and symptoms provoked by walking raise significant concern for a cerebellar infarct. The presence of vascular disease risk factors, including age, smoking, obesity, diabetes, hyperlipidemia, and hypertension, further amplifies the suspicion of cerebellar infarcts.
Approximately 75% of patients with cerebellar infarcts report experiencing dizziness in various forms, often described as vertigo or feeling like falling towards one side.[8] Many patients may complain of an inability to walk or difficulty with walking, attributed to ataxia or focal or systemic weakness. More than 50% of patients report symptoms of nausea or vomiting. The severity of symptoms is frequently more significant than evident upon examination.
Neurological findings in cerebellar strokes can manifest as localized signs like truncal or limb ataxia, as well as cranial nerve defects such as diplopia or nystagmus (particularly concerning if direction-changing or gaze-evoked, and more so if vertical or torsional), along with dysarthria. Unlike cerebral strokes, these deficits are usually ipsilesional, occurring on the same side of the patient as the cerebellar stroke. In cases of larger strokes or features of elevated intracranial pressure or brainstem compression, patients may present with lethargy, frank coma, or cardiovascular collapse, all of which indicate a poor outcome.
In some cases, the diagnosis of a cerebellar stroke is missed due to the nonspecific nature of the symptoms. The presentation largely depends on the location and extent of the lesion. Therefore, a comprehensive history and neurological exam are crucial to diagnosing cerebellar lesions.
Evaluation
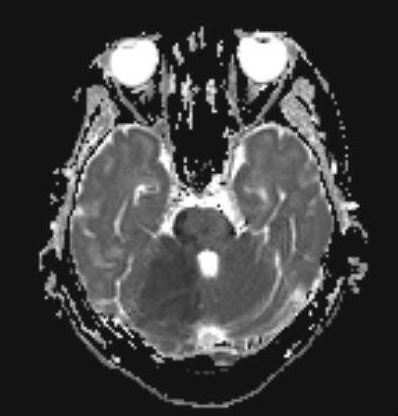
Magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI) of the brain is considered the gold standard test for diagnosing cerebellar infarction. MRI can visualize poor perfusion and signs of tissue injury, providing valuable information for accurate diagnosis (see Image. MRI Finding of Cerebellar Infarction). Additionally, magnetic resonance angiography (MRA) can localize vascular obstructions and guide endovascular treatment, especially in cases of large vessel occlusion, which is particularly beneficial in basilar artery occlusion.
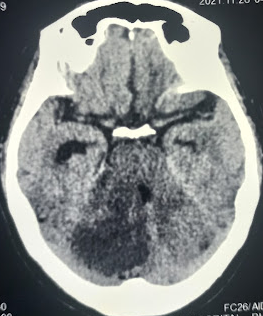
Unenhanced computed tomography (CT) imaging can assist in assessing hemorrhagic lesions and occasionally demonstrate findings suggestive of infarction (see Image. CT Image of Acute Cerebellar Infarction). However, its utility is limited by the radiopaque temporal and occipital bones surrounding the cerebellum, reducing resolution, sensitivity, and specificity compared with other brain areas.
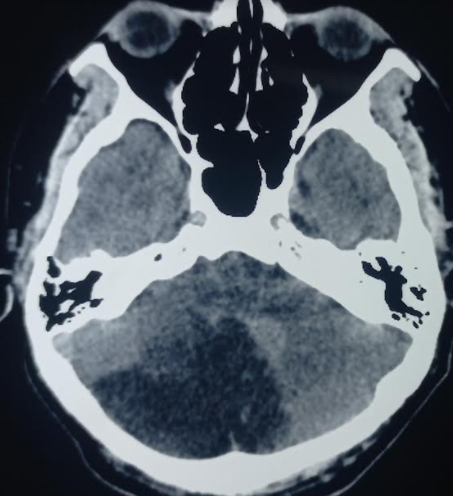
Nonetheless, unenhanced CT is usually the initial choice, partly to assess for other causes but also because it is often more readily available than MRI or MRA (see Image. Acute Cerebellar Infarction on CT Study). Enhanced CT angiogram and perfusion imaging can be viable alternatives if MRI is unavailable. In addition to a standard bedside neurological exam, the oculomotor head Head Impulse, Nystagmus, and Test of Skew (HINTS) exam can be valuable for differentiating peripheral vertigo and suggesting cerebellar infarction, especially if the "alternate eye cover test" reveals skew deviation.[9][10]
Laboratory tests, electrocardiographic assessments, echocardiographic, and electroencephalographic can be conducted to evaluate for other systemic conditions. These tests are valuable in aiding concurrent medical or surgical management.
Treatment / Management
Cerebellar infarcts are treated similarly to other ischemic cerebrovascular accidents. Patients who experience acute events with a precise onset time within 4.5 hours may be candidates for thrombolysis with recombinant tissue plasminogen activator (rtPA).[11]
The criteria for thrombolysis are mentioned later; however, diagnosing posterior infarcts can be challenging, making timely intervention difficult. Thrombectomy, similar to other strokes, remains an option. Structures in the posterior circulation, having higher white matter content and collateral flow, are believed to possess a stronger tolerance to ischemia and hypoxia than those in the forebrain.[12]
In the case of larger basilar artery occlusion, thrombectomy is often considered beyond the typical 6-hour time window, and even delayed reperfusion therapy is deemed feasible for posterior infarcts.[13] Brain imaging revealing a substantial “mismatch” between the volume of the brain infarcted and the area of decreased perfusion or a high degree of collateral circulation might prompt a more urgent thrombectomy. When reperfusion is not possible, aspirin therapy and the potential addition of another antiplatelet agent, such as clopidogrel, are indicated. Anticoagulation therapy may also be considered in patients with embolic events.
Reactive cerebral edema typically intensifies over 3 to 4 days after the initial infarct. Admission to a neurologic intensive care unit (ICU) becomes essential if it results in a worsening of neurological symptoms, ensuring thorough monitoring. Underlying preventable causes of the infarct are usually investigated during admission. Cardiac monitoring may reveal atrial fibrillation or other arrhythmias, echocardiography can reveal a patent foramen ovale or ventricular dysfunction, and blood testing can reveal diabetes mellitus or hyperlipidemia.
Cerebellar strokes, especially after the first day, tend to develop progressive edema and subsequent herniation. Therefore, admitting these patients to the ICU is critical for close monitoring. Early symptoms often include severe headache, altered mental status, vomiting, and drowsiness. In cases of large strokes with significant cerebral edema, especially if the intracranial pressure is elevated, interventions such as extraventricular drains, ventriculostomy, or decompressive suboccipital craniotomy might be necessary. Neurosurgical procedures like removal of infarcted tissue or hematoma are occasionally required. Rapidly reversible agents such as intravenous heparin should be used in these cases. Additionally, in the acute setting, temporary reduction of intracranial pressure can be achieved using agents like mannitol, hypertonic saline, or hyperventilation.
Criteria for the Treatment of Acute Ischemic Stroke with Intravenous Thrombolysis (Recombinant Tissue Plasminogen Activator or rtPA)
Inclusion Criteria
- Age ≥18 years
- Clinical diagnosis of ischemic stroke causing a noticeable neurologic deficit
- The onset of symptoms <4.5 hours before beginning treatment; if onset is not known, it is defined as the last time the patient was at his neurologic baseline
Exclusion Criteria
- Clinical
- Symptoms suggestive of subarachnoid hemorrhage
- Active internal bleeding
- Presentation suggestive of infective endocarditis
- Suspected aortic arch dissection
- Acute bleeding diathesis
- Persistent blood pressure elevation (systolic ≥185 mm Hg or diastolic ≥110 mm Hg)
- Head CT
- Evidence of hemorrhage
- Extensive regions of obvious hypodensity suggesting irreversible injury
- Patient history
- Severe head trauma or ischemic stroke in the previous 3 months
- Previous intracranial hemorrhage
- Gastrointestinal malignancy
- Intra-axial intracranial neoplasm
- Gastrointestinal hemorrhage in the last 3 weeks
- Intraspinal or intracranial surgery within the prior 3 months
- Hematologic
- Current anticoagulant use with an international normalized ratio (INR) >1.7 or prothrombin time (PT) >15 s or activated partial thromboplastin time (aPTT) >40 s
- Platelet count <100,000/mm³
- Therapeutic doses of low molecular weight heparin (LMWH) received within 24 hours; this exclusion does not apply to prophylactic doses (eg, to prevent VTE)
- Current use (ie, last dose within 48 hours in a patient with normal renal function tests) of a direct thrombin inhibitor or direct factor Xa inhibitor with evidence of an anticoagulant effect
Differential Diagnosis
The differential diagnosis for cerebellar infarction is broad and includes the following:
- Intracranial hemorrhage
- Multiple sclerosis
- Brainstem infarction [14]
- Lacunar stroke
- Middle cerebral artery stroke
- Hypertensive encephalopathy
- Migraine headache [15]
- Toxic-metabolic disturbance (eg, hypoglycemia, drug intoxication)
- Posterior reversible encephalopathy syndrome
- Seizure with postictal paresis (Todd paralysis)
Prognosis
The prognosis following a cerebellar infarction is typically comparable to that of other strokes, with larger ischemic territories being associated with higher morbidity and mortality.[16] Advances in early recognition and treatment have contributed to a decline in the overall morbidity associated with the condition over time. In general, the functional status of patients after a cerebellar infarction tends to be better than those who experience a cerebellar hemorrhage.
Complications
Delayed diagnosis and treatment of cerebellar infarction can lead to cerebral edema and, ultimately, the patient's death. However, with prompt and effective treatment and diagnosis, most patients experience positive outcomes, with functional debility being the primary complication. Cerebellar infarction is associated with acute complications during hospitalization, such as venous thromboembolism, pressure ulcers, urinary tract infections, and pneumonia.
Postoperative and Rehabilitation Care
Rehabilitation care for cerebellar stroke is a comprehensive and tailored approach aimed at optimizing recovery and restoring functional independence. Following surgical interventions, such as decompressive surgeries, patients undergo intensive monitoring in an acute care setting. Early rehabilitation initiation, often beginning in the hospital and extending to rehabilitation facilities or homes, is critical for maximizing recovery potential. A multidisciplinary team, including physical, occupational, and speech therapists, collaborates to address specific deficits associated with cerebellar stroke.
Balance and coordination training are integral components of the rehabilitation program, given the cerebellum's role in these functions. Cognitive rehabilitation may be necessary to address memory, attention, and problem-solving deficits. Assistive devices such as canes, walkers, or orthotics may be introduced to enhance mobility and safety. Home modifications are assessed and implemented to ensure a supportive environment for the patient.
Psychosocial support is an essential aspect of rehabilitation, addressing the emotional and psychological impact of the stroke on both the patient and their caregivers. Ongoing monitoring and follow-up appointments with neurologists and rehabilitation specialists allow adjustments to the care plan based on the patient's progress and emerging needs. Individualized and goal-oriented care plans, incorporating a range of rehabilitation modalities, are crucial for facilitating recovery and improving the overall quality of life for individuals who have experienced a cerebellar stroke.
Consultations
A multidisciplinary team of healthcare professionals typically sees and manages patients with a cerebellar infarct. The initial assessment and triage often occur in the emergency department, where a physician or advanced practitioner evaluates the patient. Depending on the extent of the patient's condition, the intensivist or internal medicine practitioner may oversee their care, with plans for consultation with neurology to assist in management and treatment. In cases requiring surgical intervention, neurosurgeons may be involved in procedures such as decompressive surgeries to relieve pressure on the brain. Coordination among these specialists ensures comprehensive and effective care for individuals with cerebellar infarcts.
Deterrence and Patient Education
Patient education plays a pivotal role in the secondary prevention of cerebellar stroke. It is crucial to impart information about lifestyle changes and interventions that can minimize the risk of recurrent strokes. Key aspects of patient education include encouraging smoking cessation and emphasizing the importance of maintaining optimal control over underlying comorbid conditions like diabetes and hypertension. Patients should be educated on the significance of consistent medication compliance and empowered to adopt lifestyle modifications, such as a heart-healthy diet and regular exercise, to reduce the risk of future cerebellar strokes. Healthcare providers can significantly contribute to long-term preventive measures and enhance overall health outcomes by fostering patient understanding and engagement.
Enhancing Healthcare Team Outcomes
Patients with cerebellar infarction frequently present with nonspecific symptoms, and clinical presentations can be atypical or overlap, especially in the case of hemorrhagic infarcts. Diagnosing cerebellar infarction requires a high index of suspicion and proficient diagnostic skills to ensure timely and appropriate treatment. Engaging a specialized interprofessional team consisting of clinicians, specialists, nurses, and pharmacists is essential when the disease is suspected. This collaborative approach enables a comprehensive evaluation of the case, facilitating prompt decisions on emergent treatment and determining which specialists to involve. The interprofessional paradigm is instrumental in achieving the best patient outcomes by ensuring a well-coordinated and multidisciplinary approach to managing cerebellar infarction.