Continuing Education Activity
Pleural effusion is the abnormal fluid accumulation within the pleural space, the thin cavity between the pleural layers surrounding the lungs. This condition can arise from various etiologies, ranging from heart failure and pneumonia to malignancies such as lung cancer and inflammatory disorders like lupus. Intrapleural space fluid accumulation can compress the lungs, impairing their ability to expand fully during inspiration and causing respiratory symptoms such as shortness of breath, chest pain, and cough.
Pleural effusion is a marker of increased mortality and morbidity in certain populations. Diagnosis of pleural effusion typically involves a combination of clinical evaluation, imaging studies, and diagnostic procedures such as thoracentesis. Management of pleural effusion depends on its etiology and severity and may include treating the underlying condition, draining the accumulated fluid, and addressing complications such as infection or recurrence.
This activity for healthcare professionals is designed to enhance learners' competence in evaluating and managing pleural effusion. Participants develop proficiency in diagnosing pleural effusion through clinical assessment and interpretation of imaging studies and pleural fluid analysis. Learners acquire skills in determining the appropriate management strategies, preparing them for interprofessional collaboration to improve outcomes for patients with pleural effusion.
Objectives:
Identify the cause of pleural effusion based on a patient's clinical presentation and diagnostic test results.
Differentiate between transudative and exudative pleural effusions based on laboratory pleural fluid analysis and clinical judgment.
Determine a personalized management strategy for a patient with pleural effusion.
Implement effective interprofessional communication and coordination strategies for developing diagnostic and treatment plans for patients with pleural effusion.
Introduction
A pleural effusion is an abnormal fluid accumulation within the pleural space. Under normal circumstances, a small amount of fluid is continuously produced and reabsorbed within this space to maintain lubrication and facilitate smooth movement of the lungs during respiration. However, various pathological processes can disrupt this equilibrium, leading to excessive fluid accumulation. An estimated 1.5 million patients are affected each year. Pleural effusion is a marker of increased morbidity and mortality in certain populations.
Diagnosis of pleural effusion requires combining clinical evaluation with imaging and laboratory studies, particularly pleural fluid analysis. Thoracentesis is both diagnostic and therapeutic for patients with this condition. Management strategies for this condition include treating the underlying cause, draining the accumulated fluid, and addressing complications such as infection and pleural fibrosis.
Pleural Space Anatomy
Enclosed within the bony thorax are the lungs, which expand and contract during respiration, facilitated by the pleural space. This space is between the visceral and parietal pleura and typically contains a thin layer of fluid that allows for smooth lung movements during respiration.
The pleurae consist of the visceral and parietal layers and are integral to the thoracic cavity's anatomy. The visceral pleura is tightly adherent to the lung surface and forms a continuous covering that follows the contours of the lung lobes and fissures. The visceral pleura is composed of a single mesothelial cell layer supported by connective tissue. This layer provides a frictionless surface that facilitates lung expansion and contraction during respiratory cycles. The parietal pleura lines the thoracic cavity's inner surface and is divided into the mediastinal, diaphragmatic, costal, and cervical portions. This outer layer is thicker and more vascularized than the visceral pleura and contains sensory nerve fibers contributing to pain perception.
The visceral and parietal pleurae enclose the pleural cavity, a potential space containing only minimal pleural fluid. This fluid minimizes friction between the pleural layers during respiratory movements. Gravity, ventilatory motion, and hydrostatic and oncotic pressure balance determine the volumetric fluid balance within the pleural space.[1]
Disorders affecting the pleurae, such as pleural effusion or pleurisy, can disrupt these functions, leading to respiratory symptoms and complications. Thus, a comprehensive understanding of pleural space anatomy and physiology is essential for effectively diagnosing and managing conditions involving these structures.
Etiology
Pleural effusion is caused by various conditions and is classified by Light's criteria as either a transudate or exudate. Differentiating between exudative and transudative fluids is essential in determining the underlying pathophysiology and subsequent diagnostic planning. Light's criteria serve as a reference standard for assessing pleural fluid parameters, including the quantitative ratio of pleural fluid lactate dehydrogenase (LDH) to serum LDH levels and the ratio of pleural fluid protein to serum protein levels. An exudative effusion is then diagnosed if one or more of the following criteria are met:
- Pleural fluid protein/serum protein ratio of more than 0.5
- Pleural fluid LDH/serum LDH ratio of more than 0.6
- Pleural fluid LDH is more than two-thirds of the upper limit of the normal serum LDH valuet[2][3]
If none of the above criteria is met, the fluid is considered transudative. Common causes of transudative pleural effusion include conditions that alter the hydrostatic or oncotic pressures in the pleural space, such as congestive left heart failure (CHF), nephrotic syndrome, liver cirrhosis, hypoalbuminemia, or peritoneal dialysis. Common exudative pleural effusion etiologies include pulmonary infections such as pneumonia or tuberculosis, malignancy, inflammatory disorders like pancreatitis, lupus, rheumatoid arthritis, postcardiac injury syndrome, chylothorax, hemothorax, postcoronary artery bypass grafting (post-CABG), and benign asbestos pleural effusion.
Some of the less common causes of pleural effusion are pulmonary embolism (exudative or transudative), drug-induced reactions (exudative), radiotherapy (exudative), esophageal rupture (exudative), and ovarian hyperstimulation syndrome (exudative). Drugs frequently implicated in the development of pleural effusion include methotrexate, amiodarone, phenytoin, and dasatinib.
Epidemiology
Pleural effusion is the most common condition among all pleural space diseases and affects 1.5 million patients annually in the United States. However, insufficient large-scale epidemiological studies exist surrounding its many different etiologies. Based on currently available data, the condition's occurrence rate appears to differ geographically. Notably, heart failure, malignant pleural effusion, and parapneumonic effusions account for the majority in terms of annual incidence.
Pathophysiology
The amount of fluid in the pleural space is typically around 0.1 to 0.3 ml/kg and significantly influences the hydromechanical coupling between the lung and chest wall. Pleural fluid is derived from the blood vessels of the parietal pleural surfaces, filtered out due to the systemic vessels' hydrostatic pressure. This fluid is subsequently reabsorbed through the lymphatic vessels, primarily located in the pleural cavity's dependent portions.
Accumulation of excess fluid can occur from various mechanisms. Simplistically, excessive production or decreased absorption can overwhelm the normal homeostatic mechanisms within the pleural space. Excess fluid production can follow from overfiltration from increased venous pressure, for example, in heart failure. Pleural effusion can also arise from endothelial integrity disruption by an inflammatory process, causing liquid and protein leakage. Reduced clearance, as in lymphatic blockage, can also cause an effusion.[4][5]
History and Physical
Pleural effusion symptoms can range from the absence of discernible signs to exertional breathlessness, depending on the impairment of thoracic excursion. The pathophysiology of breathlessness remains inadequately elucidated in scientific literature. The quantity of effusion seems to correlate poorly with the severity of symptoms. The impact of effusion on thoracic expansion appears to exert the most salient influence. Additional plausible etiologies include oxygen impairments and coexisting intraparenchymal diseases, such as pulmonary edema in the setting of heart failure.[6]
The patient may complain of a cough, fever, and systemic symptoms, depending on the effusion's cause. Although infrequent, effusions can occasionally attain a size significant enough to induce hemodynamic changes, imitating tamponade physiology. Patients with active pleural inflammation called "pleurisy" complain of sharp, severe, localized crescendo-decrescendo pain with breathing or coughing. Pleuritic pain tends to subside when an effusion develops. Constant pain is also a hallmark of malignant diseases like mesothelioma.
The physical examination can range from subtle to florid. Intercostal space fullness and percussion dullness are appreciated in large effusions. Auscultation reveals decreased breath sounds and tactile and vocal fremiti. Egophony is most pronounced at the effusion's superior aspect. Pleural rubs, often mistaken for coarse crackles, can be heard during active pleurisy without any effusion.
As pleural effusion results from varied diseases, history should focus on the causes of miscellaneous pulmonary and systemic effusions. Inquiring about a patient's comorbidities, medications, clinical indicators for infectious diseases, and predisposing factors to malignancies is advisable. A comprehensive physical examination should also encompass various potential diagnostic considerations. For example, CHF should be suspected in a patient with jugular venous distension, S3, and pedal edema. Advanced liver cirrhosis should be considered in individuals with ascites and caput medusae.
Evaluation
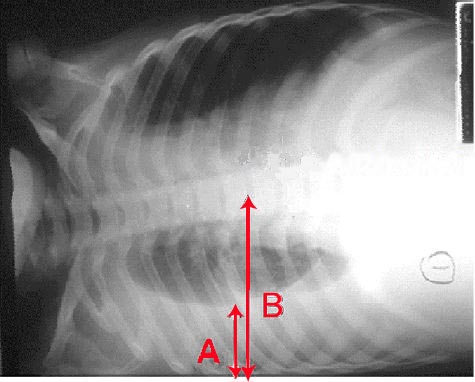
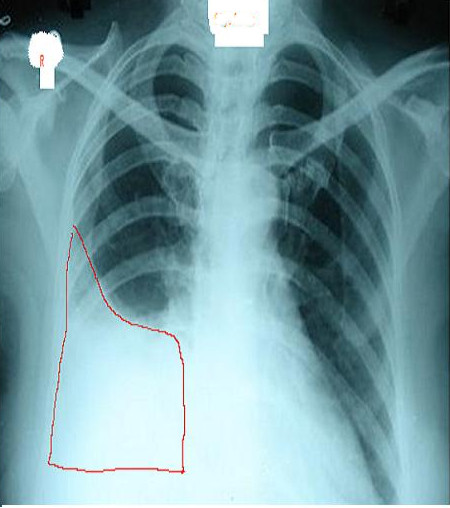
A chest radiograph is an excellent initial imaging study for identifying pleural effusion. In an upright posteroanterior (PA) view, the meniscus sign indicates the presence of a significant amount of pleural fluid, typically exceeding 200 ml and blunting the costophrenic angle (see Image. Right Lung Pleural Effusion Radiograph, Posteroanterior). However, a lateral decubitus view can detect as little as 50 ml of fluid in the pleural space (see Image. Pleural Effusion Radiograph, Lateral Decubitus).[15] Ultrasound is more sensitive and is readily available for diagnosing and confirming an effusion and planning a thoracentesis.[7][8]
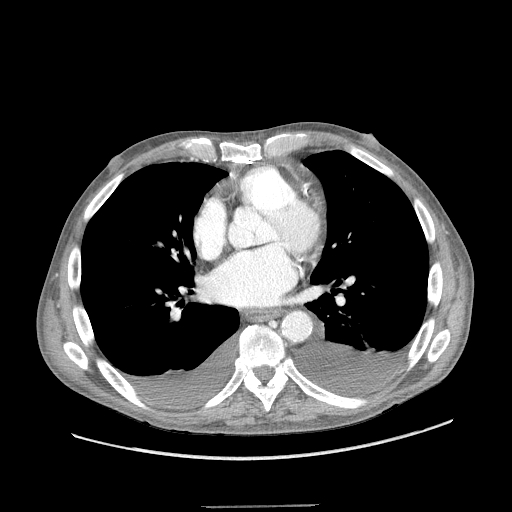
All initial effusion occurrences warrant a thoracentesis to ascertain the underlying etiology of the condition, except in instances where heart failure is confidently suspected. In such cases, a trial of diuresis may be conducted as a preliminary measure. Incorporating thoracic ultrasound in management planning is crucial. This test's prompt availability and rapid performance reduce the need for ancillary diagnostic testing such as computed tomography (CT) and offer valuable insights into fluid characteristics, which can aid in making a diagnosis (see Image. Bilateral Pleural Effusions on Chest Computed Tomography).[9] Additionally, thoracic ultrasound assists in differentiating fluid accumulation from other conditions that present as radiopaque abnormalities on chest radiographs, such as pneumonia or atelectasis.
Fluid appears hypoechoic on ultrasound. The dependent chest area and surrounding anatomical structures must be examined for better visualization. Effusion echogenicity must be noted since features such as septations may indicate a complex effusion arising from conditions like parapneumonic effusion or empyema. The hematocrit sign is characterized by smoke-like shadows, which may indicate the presence of a hemothorax. A complex-appearing fluid necessitates planning for chest tube thoracostomy (CTT).
Routinely assessed biomarkers include fluid pH, fluid and serum protein levels, fluid and serum LDH levels, fluid glucose levels, fluid cell count differential, fluid gram stain, and culture. Fluid cytology may also be examined in specific situations. Light’s criteria may then be applied to classify the fluid as an exudate or transudate. Light’s criteria should be interpreted within the clinical context since it has been reported to misclassify 25% to 30% of transudates as exudates in CHF-related pleural effusion. In such instances, obtaining a fluid proBNP or computing the albumin or protein gradient between serum and pleural fluid is helpful. A serum-to-pleural fluid albumin ratio exceeding 1.2 g/dl or a serum-to-pleural fluid total protein gradient greater than 2.5 g/dl in suspected CHF could indicate a transudative effusion.[10]
Exudative effusions necessitate further examination to determine the underlying cause. In some cases, cell count differentials can aid in narrowing down potential diagnoses. A primarily lymphocytic effusion suggests tuberculosis, post-CABG, rheumatoid arthritis, yellow nail syndrome, chylothorax, or malignancy. Eosinophilia in pleural effusion is rare and usually seen in pneumothorax, hemothorax, parasitic disease, or drug-induced effusion. Parapneumonic effusions show a neutrophil-predominant cell count. Pleural LDH values exceeding 1000 U/L are observed in tuberculosis, lymphoma, and empyema. A pH below 7.2 indicates a complex pleural effusion in the setting of pneumonia, which almost always requires CTT insertion for drainage. Low pH levels may also indicate esophageal rupture or rheumatoid arthritis.
Condition-specific markers such as acid-fast bacilli smear, tuberculosis culture, and adenosine deaminase are indicated when tuberculosis is suspected. Amylase levels are evaluated if pancreatitis-related effusion is apparent. Pleural fluid triglycerides greater than 110 mg/dL signify a chylothorax. The pleural fluid in such conditions typically has a milky-white appearance. Hemothorax can be established if the pleural fluid hematocrit is over 0.5 times that of the serum hematocrit.
Cytological testing is necessary in suspected cases of malignant pleural effusion. The sensitivity of pleural fluid cytology in diagnosing malignant effusions after the first thoracentesis is approximately 60%. However, the diagnostic yield increases with additional attempts, achieving a success rate of up to 90% after 3 samples are collected on separate days. A medical thoracoscopy with pleural biopsy is recommended after 2 successive thoracenteses if a malignant effusion is highly suspected despite negative cytology results.[11]
Invasive procedures are available to investigate exudative effusion that yields unclear results in noninvasive diagnostic tests, including closed-needle and image-guided needle biopsies, thoracoscopy, or video-assisted thoracic surgery. The clinical presentation is generally sufficient to identify a transudative effusion's cause, and additional testing is unnecessary. Nonetheless, considering less common causes, such as trapped lung, is essential where pleural elastance measurements may be required. Urinothorax is another common cause of effusion, requiring pleural and serum creatinine levels for diagnosis.
Treatment / Management
Management primarily revolves around identifying and treating the underlying cause. Pleural fluid drainage is recommended for treating symptomatic patients. In asymptomatic patients, drainage is performed only as part of the diagnostic process unless signs and symptoms of hemorrhage or infection are present. A thoracentesis in the setting of heart failure is recommended only if diuretics fail or the patient is significantly symptomatic. Chylous effusions are initially managed conservatively, though some require surgery.
Thoracentesis is a diagnostic and therapeutic procedure. Current recommendations when performing a thoracentesis include the following:
- Bedside ultrasound improves success rates and reduces the risk of pneumothorax during fluid aspiration.
- Ultrasound can detect pleural fluid sequestrations.
- Always send fluid for biochemistry, culture, and cytology.
- Use Light's criteria to distinguish exudate from transudate.
- Lymphocyte-predominant effusions are usually due to malignancy and tuberculosis.
- Check pH when aspirating pleural effusions.
- Do not inject air or local anesthetic into the sample, which may alter the fluid's pH.
- Malignant effusions can be detected on cytology (40% to 60%).
Chest tube drainage with antibiotic treatment is warranted in complex parapneumonic effusions or empyema. Small-bore drains ranging from 10 G to 14 G are equally effective as large-bore drains. Small-bore drains may even be superior due to their ease of placement and less propensity to cause patient discomfort. Intrapleural fibrinolytic and DNase administration can be utilized to enhance drainage. However, thoracoscopic decortication may be necessary when these measures fail.
Patients diagnosed with malignant effusion typically do not require frequent drainage procedures unless infection or severe symptoms arise. Individuals needing frequent drainage may opt for pleurodesis or tunneled catheter placement. No more than 1,500 ml of fluid should be extracted in a single session, as doing so may produce reexpansion pulmonary edema.
Differential Diagnosis
The differential diagnosis of pleural effusion includes the following:
- Diaphragmatic paralysis appears as a dense and radiopaque substance on radiographs and can sometimes be mistaken for pleural effusion. However, a distinguishing feature is the presence of an elevated diaphragm with a smooth surface and a narrow costophrenic sulcus.
- Lobar pneumonia can appear in a plain radiograph as a uniform opacification, which can be difficult to differentiate from an effusion. Ultrasonography can distinguish between these conditions.
- A substantial loss in volume due to lobar collapse can result in complete opacification on a plain radiograph that may be mistaken for an effusion.
A thorough clinical evaluation and appropriate use of imaging and laboratory tests can differentiate pleural effusion from these conditions.
Prognosis
Limited data are available regarding the prognostic factors associated with pleural effusion. While it is already established that individuals with malignant effusions carry a dismal prognosis, the mortality rate for those with nonmalignant effusions has not been extensively studied.[7] Nevertheless, several prospective studies have indicated that effusion is a potential indicator of increased mortality.
Complications
Pleural effusion, while often manageable, can lead to various complications. One is empyema, characterized by accumulated infected fluid within the pleural space. Empyema can result in systemic infection, sepsis, and respiratory compromise, necessitating aggressive treatment with antibiotics and, in severe cases, drainage procedures such as thoracentesis or CTT insertion.
Another potential complication of pleural effusion is pleural thickening from fibrous adhesions, which can occur due to chronic inflammation or repeated effusion episodes. Pleural thickening can lead to decreased lung expansion, impaired respiratory function, and restrictive lung disease. Pulmonary rehabilitation or steroids may be enough for some patients. In others, surgical procedures such as thoracoscopic or open decortication can remove the fibrous tissue and release the adhesions, allowing for improved lung expansion and respiratory function.
Consultations
The following consultations are recommended:
- Pulmonology, to assess the severity of pleural effusion, determine treatment options, and coordinate care with other team members.
- Thoracic surgery, to perform surgical procedures involving the chest, including interventions that address complicated pleural effusion.
These medical specialists can help provide comprehensive care tailored to the patient's needs.
Deterrence and Patient Education
Primary preventive measures for pleural effusion involve preventing the development of conditions that can lead to its occurrence. These preventive actions include engaging in regular physical activity, maintaining a healthy diet, limiting alcohol consumption, and abstaining from smoking. Vaccinating against infectious diseases like pneumonia and influenza can also help prevent respiratory infections that may contribute to pleural effusion. Occupational safety measures, such as proper respiratory protection in workplaces with potential exposure to hazardous materials, can also reduce the risk of developing pleural effusion due to occupational lung diseases.
Secondary preventive measures focus on early detection and intervention to prevent complications associated with pleural effusion. Regular health check-ups, particularly for individuals with underlying risk factors such as heart or lung conditions, can facilitate early identification of symptoms suggestive of pleural effusion. Diagnostic tests like chest x-rays and CT scans may be recommended for individuals with respiratory symptoms or those at high risk for pleural effusion. Timely treatment of underlying medical conditions, such as heart failure or pneumonia, can help prevent the progression of these conditions to pleural effusion. Appropriate management of existing pleural effusions, including drainage procedures and addressing underlying causes, can prevent complications such as infection or recurrence.
Pearls and Other Issues
Pleural effusion is characterized by an abnormal accumulation of fluid in the pleural space, which can result from various underlying conditions such as heart failure, infection, or malignancy. Diagnosis typically involves imaging studies like radiography and CT scanning, followed by thoracentesis for fluid analysis. Treatment depends on the cause and severity of symptoms, ranging from addressing the underlying condition to draining the fluid and performing pleurodesis in some cases. Complications, including respiratory compromise and infection, require prompt recognition and management. Multidisciplinary collaboration among healthcare specialties is often necessary for comprehensive care.
Enhancing Healthcare Team Outcomes
The interprofessional team managing pleural effusions comprises specialists from diverse fields collaborating to ensure comprehensive patient care. Pulmonologists lead in diagnosing and treating respiratory conditions, assessing effusion severity, and coordinating care plans. These specialists work closely with cardiothoracic surgeons who perform procedures to treat complex cases or prevent recurrence. Radiologists provide expertise in interpreting imaging studies, aiding in accurate diagnosis and treatment planning. Pathologists analyze fluid and tissue samples to confirm diagnoses and rule out malignancies or infections. Other specialists that may also be involved include but are not limited to oncologists, infectious disease specialists, cardiologists, interventional radiologists, and hepatologists, whose expertise may be required to treat the underlying cause of the pleural effusion.
Respiratory therapists offer respiratory care and assist in managing symptoms through techniques like breathing exercises and oxygen therapy. Nurses are crucial to patient education, care coordination, and monitoring patient progress throughout treatment. Physical therapists develop tailored exercise programs to optimize lung function and mobility, particularly following procedures. By working collaboratively, this interprofessional team ensures that patients with pleural effusions receive comprehensive, personalized care to enhance outcomes and quality of life.[8][9]