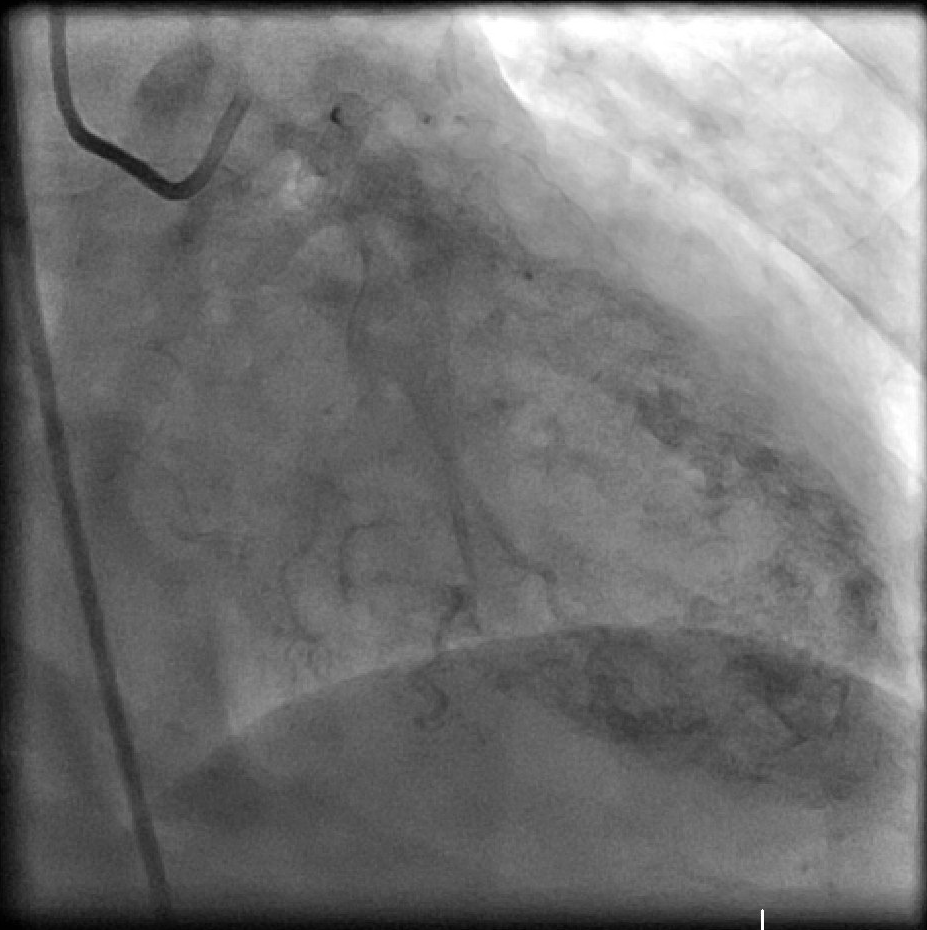
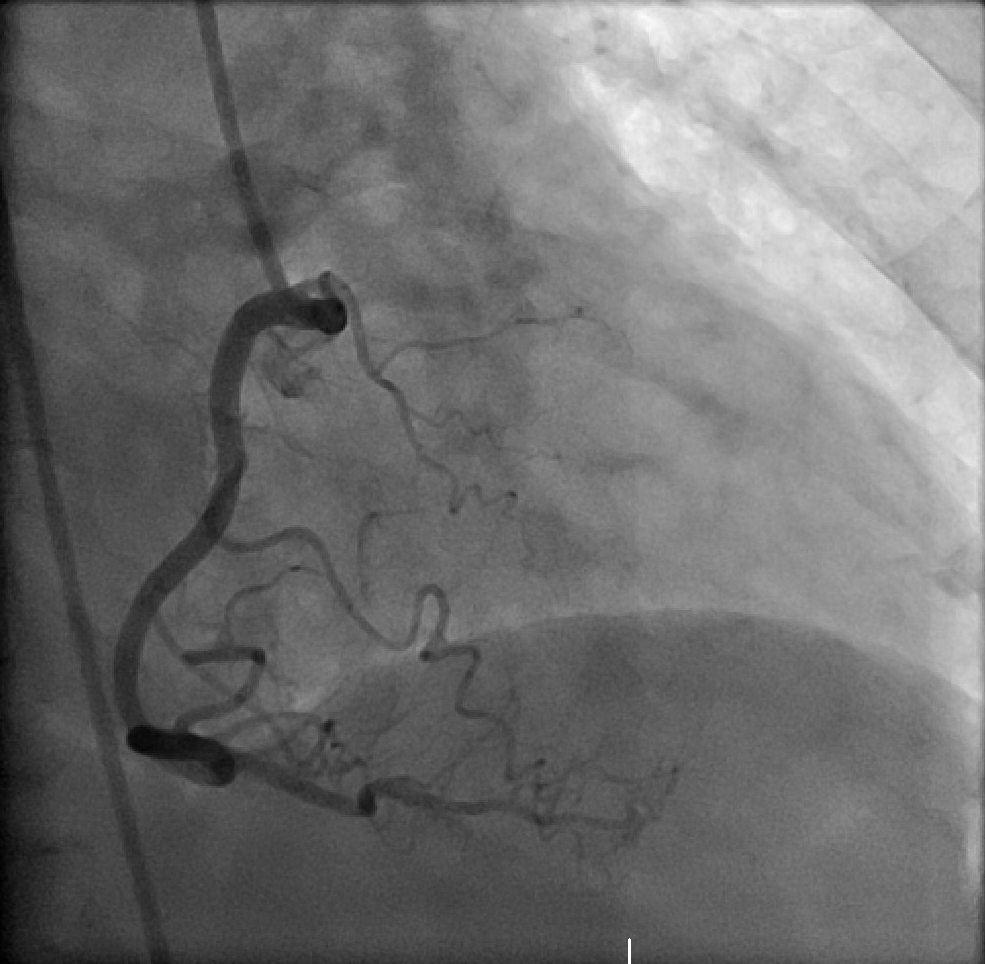
[1]
Fernandes ED, Kadivar H, Hallman GL, Reul GJ, Ott DA, Cooley DA. Congenital malformations of the coronary arteries: the Texas Heart Institute experience. The Annals of thoracic surgery. 1992 Oct:54(4):732-40
[PubMed PMID: 1417232]
[2]
Olearchyk AS, Runk DM, Alavi M, Grosso MA. Congenital bilateral coronary-to-pulmonary artery fistulas. The Annals of thoracic surgery. 1997 Jul:64(1):233-5
[PubMed PMID: 9236369]
[3]
Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature (Austin, Tex.). 2016 Jan-Mar:3(1):92-103. doi: 10.1080/23328940.2015.1088502. Epub 2015 Oct 12
[PubMed PMID: 27227081]
[4]
Maron BJ,Shirani J,Poliac LC,Mathenge R,Roberts WC,Mueller FO, Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996 Jul 17;
[PubMed PMID: 8667563]
[5]
Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. Journal of the American College of Cardiology. 2000 May:35(6):1493-501
[PubMed PMID: 10807452]
[6]
Cox ID, Bunce N, Fluck DS. Failed sudden cardiac death in a patient with an anomalous origin of the right coronary artery. Circulation. 2000 Sep 19:102(12):1461-2
[PubMed PMID: 10993868]
[7]
Van Camp SP, Bloor CM, Mueller FO, Cantu RC, Olson HG. Nontraumatic sports death in high school and college athletes. Medicine and science in sports and exercise. 1995 May:27(5):641-7
[PubMed PMID: 7674867]
[8]
Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. International journal of cardiology. 2006 Feb 8:107(1):7-10
[PubMed PMID: 16125261]
[9]
Lin FC, Chang HJ, Chern MS, Wen MS, Yeh SJ, Wu D. Multiplane transesophageal echocardiography in the diagnosis of congenital coronary artery fistula. American heart journal. 1995 Dec:130(6):1236-44
[PubMed PMID: 7484775]
[10]
Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. The International journal of angiology : official publication of the International College of Angiology, Inc. 2014 Mar:23(1):1-10. doi: 10.1055/s-0033-1349162. Epub
[PubMed PMID: 24940026]
[11]
Ogden JA. Congenital anomalies of the coronary arteries. The American journal of cardiology. 1970 Apr:25(4):474-9
[PubMed PMID: 5438244]
[12]
Yun G,Nam TH,Chun EJ, Coronary Artery Fistulas: Pathophysiology, Imaging Findings, and Management. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 May-Jun;
[PubMed PMID: 29601265]
[13]
Mangukia CV. Coronary artery fistula. The Annals of thoracic surgery. 2012 Jun:93(6):2084-92. doi: 10.1016/j.athoracsur.2012.01.114. Epub 2012 May 5
[PubMed PMID: 22560322]
[14]
Ata Y, Turk T, Bicer M, Yalcin M, Ata F, Yavuz S. Coronary arteriovenous fistulas in the adults: natural history and management strategies. Journal of cardiothoracic surgery. 2009 Nov 6:4():62. doi: 10.1186/1749-8090-4-62. Epub 2009 Nov 6
[PubMed PMID: 19891792]
[15]
Dimitrakakis G, Von Oppell U, Luckraz H, Groves P. Surgical repair of triple coronary-pulmonary artery fistulae with associated atrial septal defect and aortic valve regurgitation. Interactive cardiovascular and thoracic surgery. 2008 Oct:7(5):933-4. doi: 10.1510/icvts.2008.181388. Epub 2008 Jun 10
[PubMed PMID: 18544587]
[16]
Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. The Annals of thoracic surgery. 2000 Apr:69(4 Suppl):S270-97
[PubMed PMID: 10798435]
[17]
Latson LA. Coronary artery fistulas: how to manage them. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2007 Jul 1:70(1):110-6
[PubMed PMID: 17420995]
[18]
Shiga Y, Tsuchiya Y, Yahiro E, Kodama S, Kotaki Y, Shimoji E, Fukuda N, Morito N, Urata M, Saito N, Niimura H, Mihara H, Yamanouchi Y, Urata H. Left main coronary trunk connecting into right atrium with an aneurysmal coronary artery fistula. International journal of cardiology. 2008 Jan 11:123(2):e28-30
[PubMed PMID: 17306898]
[20]
Angelini P. Coronary artery anomalies--current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Texas Heart Institute journal. 2002:29(4):271-8
[PubMed PMID: 12484611]
[21]
Krishnamoorthy KM, Rao S. Transesophageal echocardiography for the diagnosis of coronary arteriovenous fistula. International journal of cardiology. 2004 Aug:96(2):281-3
[PubMed PMID: 15262046]
[22]
Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Chen YY. Identification of a coronary-to-bronchial-artery communication with MDCT shows the diagnostic potential of this new technology: case report and review. Journal of thoracic imaging. 2007 Aug:22(3):274-6
[PubMed PMID: 17721342]
Level 3 (low-level) evidence
[23]
Parga JR, Ikari NM, Bustamante LN, Rochitte CE, de Avila LF, Oliveira SA. Case report: MRI evaluation of congenital coronary artery fistulae. The British journal of radiology. 2004 Jun:77(918):508-11
[PubMed PMID: 15151973]
Level 3 (low-level) evidence
[24]
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008 Dec 2:118(23):2395-451. doi: 10.1161/CIRCULATIONAHA.108.190811. Epub 2008 Nov 7
[PubMed PMID: 18997168]
Level 1 (high-level) evidence
[25]
Schneider DJ. The patent ductus arteriosus in term infants, children, and adults. Seminars in perinatology. 2012 Apr:36(2):146-53. doi: 10.1053/j.semperi.2011.09.025. Epub
[PubMed PMID: 22414886]
[26]
Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2010 Apr:18(4):209-11
[PubMed PMID: 20428420]
[27]
Ahn S, Han J, Kim HK, Kim TS. Pulmonary Arteriovenous Fistula: Clinical and Histologic Spectrum of Four Cases. Journal of pathology and translational medicine. 2016 Sep:50(5):390-3. doi: 10.4132/jptm.2016.04.18. Epub 2016 May 9
[PubMed PMID: 27156513]
Level 3 (low-level) evidence
[28]
Cottin V, Chinet T, Lavolé A, Corre R, Marchand E, Reynaud-Gaubert M, Plauchu H, Cordier JF, Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine. 2007 Jan:86(1):1-17. doi: 10.1097/MD.0b013e31802f8da1. Epub
[PubMed PMID: 17220751]
[29]
Santhirapala V, Chamali B, McKernan H, Tighe HC, Williams LC, Springett JT, Bellenberg HR, Whitaker AJ, Shovlin CL. Orthodeoxia and postural orthostatic tachycardia in patients with pulmonary arteriovenous malformations: a prospective 8-year series. Thorax. 2014 Nov:69(11):1046-7. doi: 10.1136/thoraxjnl-2014-205289. Epub 2014 Apr 8
[PubMed PMID: 24713588]
[30]
Hanley M, Ahmed O, Chandra A, Gage KL, Gerhard-Herman MD, Ginsburg M, Gornik HL, Johnson PT, Oliva IB, Ptak T, Steigner ML, Strax R, Rybicki FJ, Dill KE. ACR Appropriateness Criteria Clinically Suspected Pulmonary Arteriovenous Malformation. Journal of the American College of Radiology : JACR. 2016 Jul:13(7):796-800. doi: 10.1016/j.jacr.2016.03.020. Epub 2016 May 19
[PubMed PMID: 27209598]
[31]
Ghaderian M. Aortopulmonary window in infants. Heart views : the official journal of the Gulf Heart Association. 2012 Jul:13(3):103-6. doi: 10.4103/1995-705X.102153. Epub
[PubMed PMID: 23181179]
[32]
Pfannschmidt J,Ruskowski H,de Vivie ER, [Bland-White-Garland syndrome. Clinical aspects, diagnosis, therapy]. Klinische Padiatrie. 1992 Sep-Oct;
[PubMed PMID: 1405418]
[33]
Shaikh AH, Hanif B, Khan G, Hasan K. Supracristal ventricular septal defect with severe right coronary cusp prolapse. JPMA. The Journal of the Pakistan Medical Association. 2011 Jun:61(6):605-7
[PubMed PMID: 22204223]
[34]
Peter AA, Ferreira AC, Zelnick K, Sangosanya A, Chirinos J, de Marchena E. Internal mammary artery to pulmonary vasculature fistula--case series. International journal of cardiology. 2006 Mar 22:108(1):135-8
[PubMed PMID: 16516712]
Level 2 (mid-level) evidence
[35]
Sigler L, Gutiérrez-Carreño R, Martínez-López C, Lizola RI, Sánchez-Fabela C. Aortocava fistula: experience with five patients. Vascular surgery. 2001 May-Jun:35(3):207-12
[PubMed PMID: 11452347]
[36]
Kiefer TL, Crowley AL, Jaggers J, Harrison JK. Coronary arteriovenous fistulae: the complexity of coronary artery-to-coronary sinus connections. Texas Heart Institute journal. 2012:39(2):218-22
[PubMed PMID: 22740735]