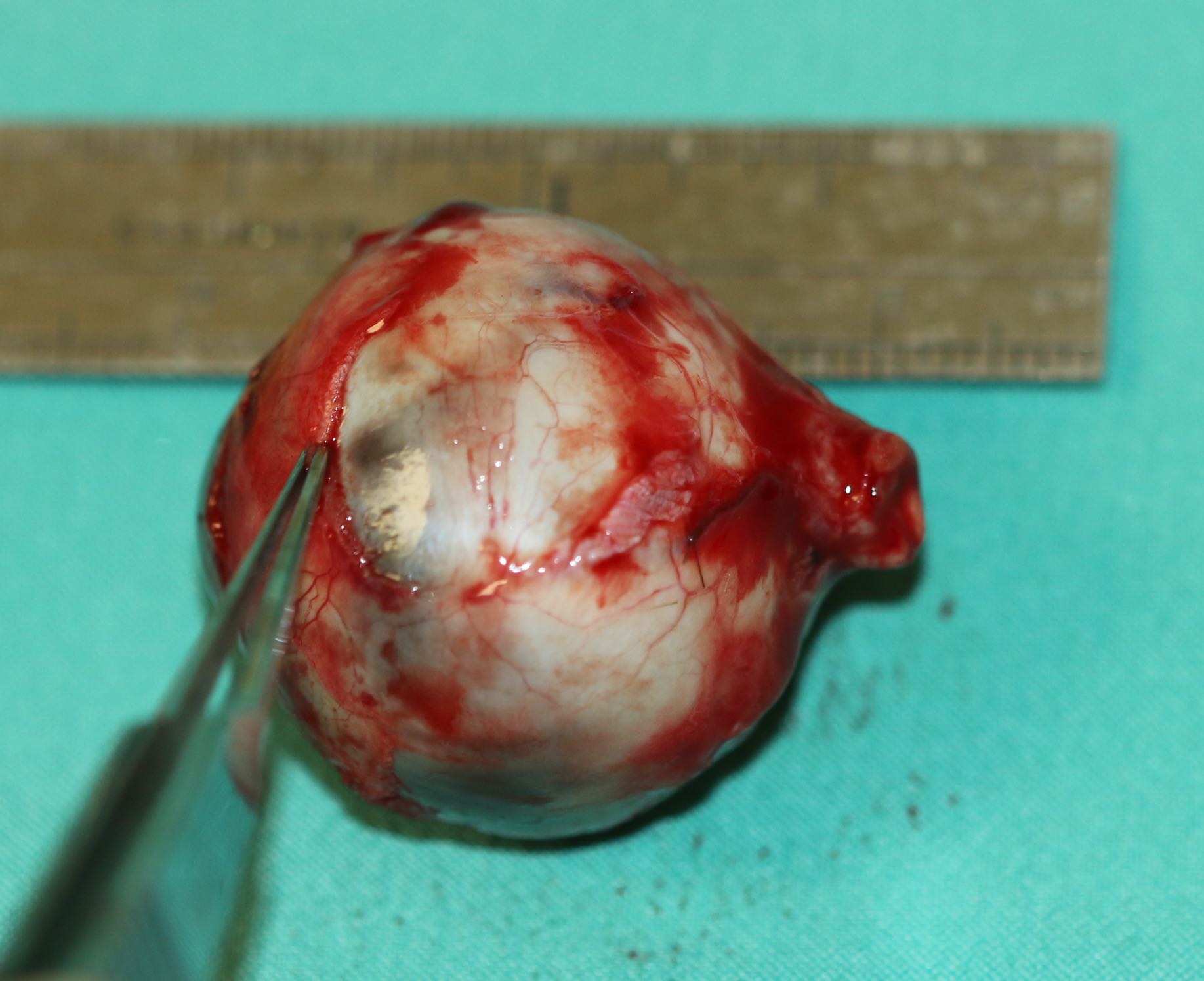
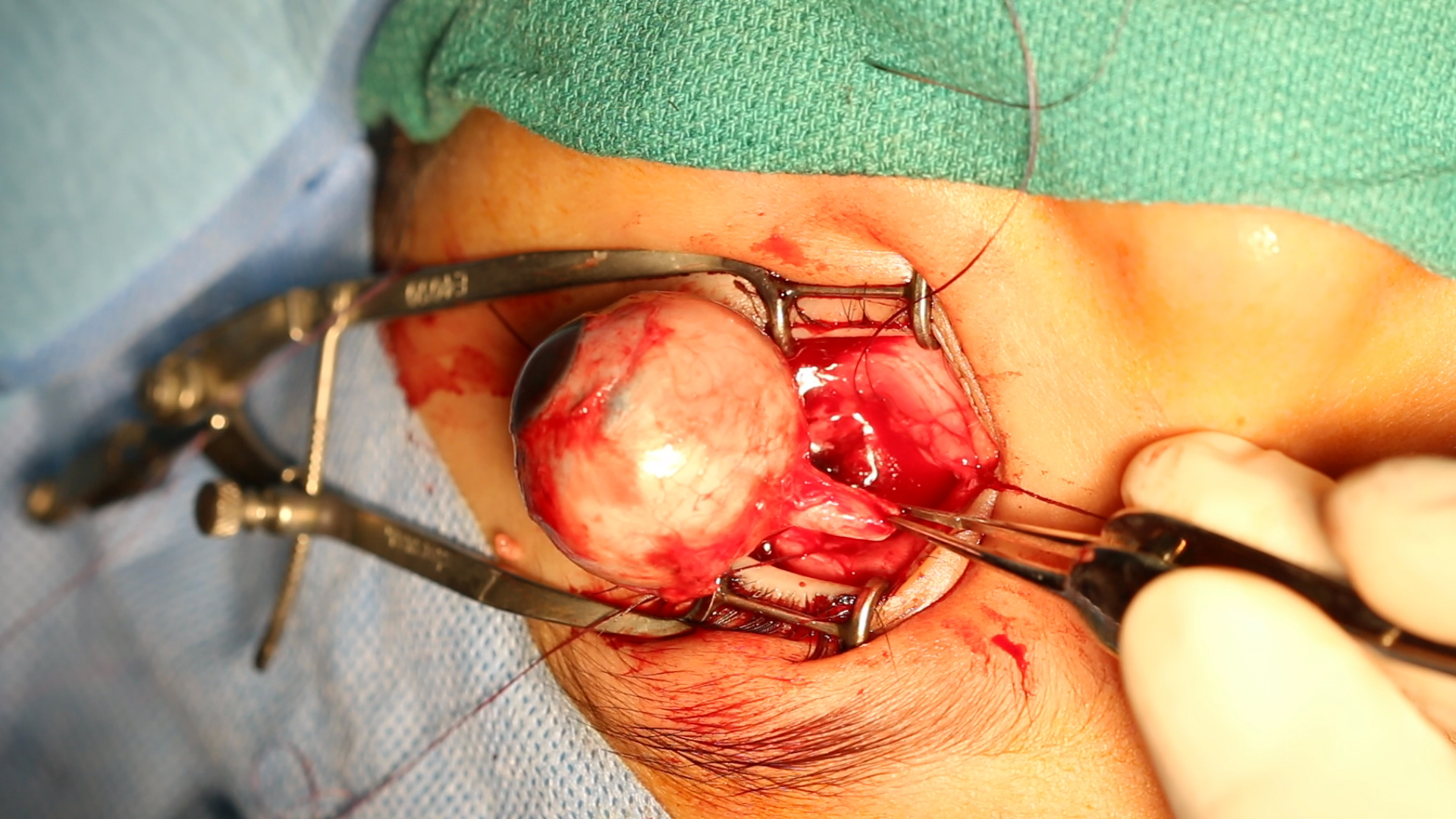
[1]
Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Survey of ophthalmology. 2000 Jan-Feb:44(4):277-301
[PubMed PMID: 10667436]
Level 3 (low-level) evidence
[2]
Jovanovic N, Carniciu AL, Russell WW, Jarocki A, Kahana A. Reconstruction of the Orbit and Anophthalmic Socket Using the Dermis Fat Graft: A Major Review. Ophthalmic plastic and reconstructive surgery. 2020 Nov/Dec:36(6):529-539. doi: 10.1097/IOP.0000000000001610. Epub
[PubMed PMID: 32134765]
[3]
Borrelli M, Geerling G, Spaniol K, Witt J. Eye Socket Regeneration and Reconstruction. Current eye research. 2020 Mar:45(3):253-264. doi: 10.1080/02713683.2020.1712423. Epub 2020 Jan 10
[PubMed PMID: 31910675]
[4]
Tao JP, Aakalu VK, Wladis EJ, Sobel RK, Freitag SK, Foster JA, Yen MT. Bioengineered Acellular Dermal Matrix Spacer Grafts for Lower Eyelid Retraction Repair: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020 May:127(5):689-695. doi: 10.1016/j.ophtha.2019.11.011. Epub 2019 Dec 30
[PubMed PMID: 31899031]
[5]
Kashkouli MB, Zolfaghari R, Es'haghi A, Amirsardari A, Abtahi MB, Karimi N, Alemzadeh A, Aghamirsalim M. Tear Film, Lacrimal Drainage System, and Eyelid Findings in Subjects With Anophthalmic Socket Discharge. American journal of ophthalmology. 2016 May:165():33-8. doi: 10.1016/j.ajo.2016.02.016. Epub 2016 Feb 28
[PubMed PMID: 26930225]
[6]
Rokohl AC, Trester M, Guo Y, Adler W, Jaeger VK, Loreck N, Mor JM, Pine KR, Heindl LM. Dry anophthalmic socket syndrome - Standardized clinical evaluation of symptoms and signs. The ocular surface. 2020 Jul:18(3):453-459. doi: 10.1016/j.jtos.2020.05.001. Epub 2020 May 6
[PubMed PMID: 32387569]
[7]
Custer PL, Trinkaus KM. Volumetric determination of enucleation implant size. American journal of ophthalmology. 1999 Oct:128(4):489-94
[PubMed PMID: 10577591]
[8]
Kaltreider SA, Jacobs JL, Hughes MO. Predicting the ideal implant size before enucleation. Ophthalmic plastic and reconstructive surgery. 1999 Jan:15(1):37-43
[PubMed PMID: 9949428]
[9]
Thaller VT. Enucleation volume measurement. Ophthalmic plastic and reconstructive surgery. 1997 Mar:13(1):18-20
[PubMed PMID: 9076778]
[10]
Fountain TR, Goldberger S, Murphree AL. Orbital development after enucleation in early childhood. Ophthalmic plastic and reconstructive surgery. 1999 Jan:15(1):32-6
[PubMed PMID: 9949427]
[11]
Kaltreider SA, Peake LR, Carter BT. Pediatric enucleation: analysis of volume replacement. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Mar:119(3):379-84
[PubMed PMID: 11231771]
[12]
De Potter P, Shields CL, Shields JA, Singh AD. Use of the hydroxyapatite ocular implant in the pediatric population. Archives of ophthalmology (Chicago, Ill. : 1960). 1994 Feb:112(2):208-12
[PubMed PMID: 8311774]
[13]
Dharmasena A, Keenan T, Goldacre R, Hall N, Goldacre MJ. Trends over time in the incidence of congenital anophthalmia, microphthalmia and orbital malformation in England: database study. The British journal of ophthalmology. 2017 Jun:101(6):735-739. doi: 10.1136/bjophthalmol-2016-308952. Epub 2016 Sep 6
[PubMed PMID: 27601422]
[14]
Shah M, Sun L, Elmann S, Vrcek I, Mancini R, Kim HJ, Carrasco J, Shinder R. Self-inflicted enucleations: Clinical features of seven cases. Orbit (Amsterdam, Netherlands). 2017 Jun:36(3):154-158. doi: 10.1080/01676830.2017.1279670. Epub 2017 Mar 3
[PubMed PMID: 28594303]
Level 3 (low-level) evidence
[15]
Gündüz AK, Mirzayev I, Temel E, Ünal E, Taçyıldız N, Dinçaslan H, Köse SK, Özalp Ateş FS, Işık MU. A 20-year audit of retinoblastoma treatment outcomes. Eye (London, England). 2020 Oct:34(10):1916-1924. doi: 10.1038/s41433-020-0898-9. Epub 2020 May 6
[PubMed PMID: 32376976]
[16]
Maheshwari A, Finger PT. Cancers of the eye. Cancer metastasis reviews. 2018 Dec:37(4):677-690. doi: 10.1007/s10555-018-9762-9. Epub
[PubMed PMID: 30203109]
[17]
Bergeron E, Lihimdi N, Bergeron D, Landreville S. Orbital recurrence of iris melanoma 21 years after enucleation. BMJ case reports. 2017 Sep 7:2017():. pii: bcr-2017-221137. doi: 10.1136/bcr-2017-221137. Epub 2017 Sep 7
[PubMed PMID: 28882848]
Level 3 (low-level) evidence
[18]
Tan XL, Seen S, Dutta Majumder P, Ganesh SK, Agarwal M, Soni A, Biswas J, Aggarwal K, Mahendradas P, Gupta V, Ling HS, Teoh S, Pavesio C, Agrawal R. Analysis of 130 Cases of Sympathetic Ophthalmia - A Retrospective Multicenter Case Series. Ocular immunology and inflammation. 2019:27(8):1259-1266. doi: 10.1080/09273948.2018.1517894. Epub 2018 Sep 12
[PubMed PMID: 30207811]
Level 2 (mid-level) evidence
[19]
Yousuf SJ, Jones LS, Kidwell ED Jr. Enucleation and evisceration: 20 years of experience. Orbit (Amsterdam, Netherlands). 2012 Aug:31(4):211-5. doi: 10.3109/01676830.2011.639477. Epub 2012 May 29
[PubMed PMID: 22642653]
[20]
Zheng C, Wu AY. Enucleation versus evisceration in ocular trauma: a retrospective review and study of current literature. Orbit (Amsterdam, Netherlands). 2013 Dec:32(6):356-61. doi: 10.3109/01676830.2013.764452. Epub 2013 Aug 2
[PubMed PMID: 23909276]
Level 2 (mid-level) evidence
[21]
Ang MJ, Silkiss RZ. The Use of Long-Acting Liposomal Bupivacaine (Exparel) for Postoperative Pain Control Following Enucleation or Evisceration. Ophthalmic plastic and reconstructive surgery. 2018 Nov/Dec:34(6):599. doi: 10.1097/IOP.0000000000001218. Epub
[PubMed PMID: 30418397]
[22]
Sobti MM, Shams F, Jawaheer L, Cauchi P, Chadha V. Unwrapped hydroxyapatite orbital implants: our experience in 347 cases. Eye (London, England). 2020 Apr:34(4):675-682. doi: 10.1038/s41433-019-0571-3. Epub 2019 Sep 16
[PubMed PMID: 31527766]
Level 3 (low-level) evidence
[23]
Wladis EJ, Aakalu VK, Sobel RK, Yen MT, Bilyk JR, Mawn LA. Orbital Implants in Enucleation Surgery: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018 Feb:125(2):311-317. doi: 10.1016/j.ophtha.2017.08.006. Epub 2017 Sep 9
[PubMed PMID: 28899574]
[24]
Mourits DL, Hartong DT, Bosscha MI, Kloos RJ, Moll AC. Worldwide enucleation techniques and materials for treatment of retinoblastoma: an international survey. PloS one. 2015:10(3):e0121292. doi: 10.1371/journal.pone.0121292. Epub 2015 Mar 13
[PubMed PMID: 25767872]
Level 3 (low-level) evidence
[25]
Ramey N, Gupta D, Price K, Husain A, Richard M, Woodward J. Comparison of complication rates of porous anophthalmic orbital implants. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2011 Sep-Oct:42(5):434-40. doi: 10.3928/15428877-20110812-03. Epub
[PubMed PMID: 21899247]
[26]
Hintschich C, Raithel E, Craig GT, Bernatzky G, Alzner E, Brook IM, Collin R. Glass-ionomer cement: evaluation as an orbital implant. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1999 Feb:237(2):169-74
[PubMed PMID: 9987636]
[27]
Rose GE, Collin R. Dermofat grafts to the extraconal orbital space. The British journal of ophthalmology. 1992 Jul:76(7):408-11
[PubMed PMID: 1627511]
[28]
Pelton RW, Patel BC. Superomedial lid crease approach to the medial intraconal space: a new technique for access to the optic nerve and central space. Ophthalmic plastic and reconstructive surgery. 2001 Jul:17(4):241-53
[PubMed PMID: 11476174]
[29]
Jordan DR, Brownstein S, Jolly SS. Abscessed hydroxyapatite orbital implants. A report of two cases. Ophthalmology. 1996 Nov:103(11):1784-7
[PubMed PMID: 8942870]
Level 3 (low-level) evidence
[30]
Soparkar CN, Patrinely JR. Abscessed hydroxyapatite orbital implants. Ophthalmology. 1997 Jul:104(7):1059
[PubMed PMID: 9224451]
[31]
Smit TJ, Koornneef L, Zonneveld FW, Groet E, Otto AJ. Computed tomography in the assessment of the postenucleation socket syndrome. Ophthalmology. 1990 Oct:97(10):1347-51
[PubMed PMID: 2243686]
[32]
Rose GE, Sigurdsson H, Collin R. The volume-deficient orbit: clinical characteristics, surgical management, and results after extraperiorbital implantation of Silastic block. The British journal of ophthalmology. 1990 Sep:74(9):545-50
[PubMed PMID: 2393645]
[33]
Bosniak SL. Dermis-fat orbital implantation and complex socket deformities. Advances in ophthalmic plastic and reconstructive surgery. 1992:9():131-41
[PubMed PMID: 1457056]
Level 3 (low-level) evidence