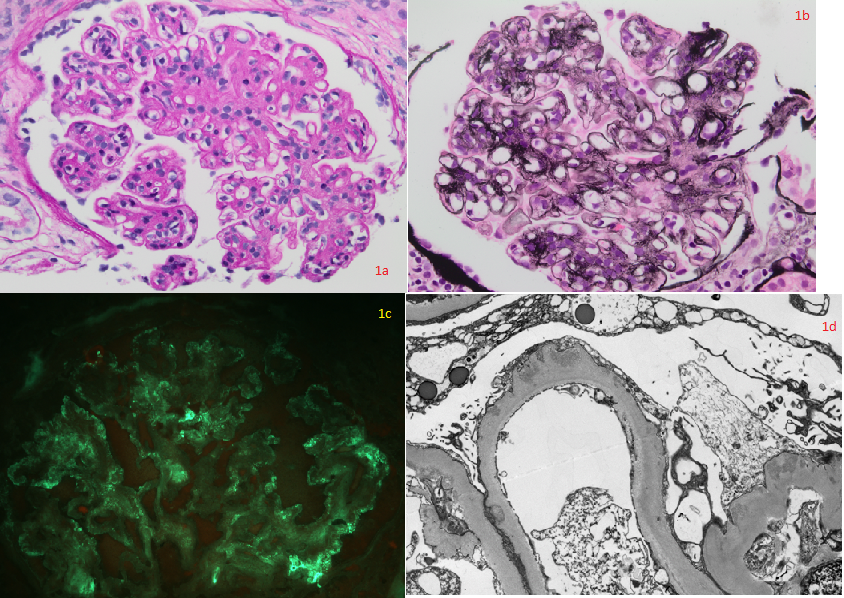
[1]
Rasmussen N, Wiik A, Jayne DR. A historical essay on detection of anti-neutrophil cytoplasmic antibodies. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Apr:30 Suppl 1():i8-13. doi: 10.1093/ndt/gfv070. Epub
[PubMed PMID: 25805749]
[2]
Wesolowski LG, Wroblewski K, Bennett SB, Parker MM, Hagan C, Ethridge SF, Rhodes J, Sullivan TJ, Ignacio-Hernando I, Werner BG, Owen SM. Nucleic acid testing by public health referral laboratories for public health laboratories using the U.S. HIV diagnostic testing algorithm. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2015 Apr:65():6-10. doi: 10.1016/j.jcv.2015.01.017. Epub 2015 Jan 24
[PubMed PMID: 25766979]
[3]
Muñoz A, Kirby AJ, He YD, Margolick JB, Visscher BR, Rinaldo CR, Kaslow RA, Phair JP. Long-term survivors with HIV-1 infection: incubation period and longitudinal patterns of CD4+ lymphocytes. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1995 Apr 15:8(5):496-505
[PubMed PMID: 7697447]
[4]
Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. The New England journal of medicine. 1998 Jul 2:339(1):33-9
[PubMed PMID: 9647878]
[5]
Tindall B, Barker S, Donovan B, Barnes T, Roberts J, Kronenberg C, Gold J, Penny R, Cooper D. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Archives of internal medicine. 1988 Apr:148(4):945-9
[PubMed PMID: 3258508]
[6]
Jansen TL, van Houte D, de Vries T, Wolthuis A. ANCA seropositivity in HIV: a serological pitfall. The Netherlands journal of medicine. 2005 Jul-Aug:63(7):270-4
[PubMed PMID: 16093579]
[7]
Ross MJ, Bruggeman LA, Wilson PD, Klotman PE. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. Journal of the American Society of Nephrology : JASN. 2001 Dec:12(12):2645-2651. doi: 10.1681/ASN.V12122645. Epub
[PubMed PMID: 11729233]
[8]
Winston JA. HIV and CKD epidemiology. Advances in chronic kidney disease. 2010 Jan:17(1):19-25. doi: 10.1053/j.ackd.2009.08.006. Epub
[PubMed PMID: 20005485]
Level 3 (low-level) evidence
[9]
Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science (New York, N.Y.). 2010 Aug 13:329(5993):841-5. doi: 10.1126/science.1193032. Epub 2010 Jul 15
[PubMed PMID: 20647424]
[10]
Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D'Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. The New England journal of medicine. 2001 Jun 28:344(26):1979-84
[PubMed PMID: 11430327]
[11]
Foy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, Atta MG. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clinical journal of the American Society of Nephrology : CJASN. 2013 Sep:8(9):1524-32. doi: 10.2215/CJN.10991012. Epub 2013 May 16
[PubMed PMID: 23685946]
[12]
D'Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Seminars in nephrology. 1998 Jul:18(4):406-21
[PubMed PMID: 9692353]
[13]
Lucas GM,Ross MJ,Stock PG,Shlipak MG,Wyatt CM,Gupta SK,Atta MG,Wools-Kaloustian KK,Pham PA,Bruggeman LA,Lennox JL,Ray PE,Kalayjian RC, Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Nov 1
[PubMed PMID: 25234519]
Level 1 (high-level) evidence
[14]
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A, PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. The New England journal of medicine. 2008 Feb 7:358(6):568-79. doi: 10.1056/NEJMoa0706135. Epub
[PubMed PMID: 18256392]
[15]
Hashemi MS, Irajpour A, Abazari P. Improving Quality of Care in Hemodialysis: a Content Analysis. Journal of caring sciences. 2018 Sep:7(3):149-155. doi: 10.15171/jcs.2018.024. Epub 2018 Sep 1
[PubMed PMID: 30283760]
Level 2 (mid-level) evidence
[16]
Elgalib A, Al-Sawafi H, Kamble B, Al-Harthy S, Al-Sariri Q. Multidisciplinary care model for HIV improves treatment outcome: a single-centre experience from the Middle East. AIDS care. 2018 Sep:30(9):1114-1119. doi: 10.1080/09540121.2018.1479028. Epub 2018 May 24
[PubMed PMID: 29792340]
[17]
Molas E, Luque S, Retamero A, Echeverría-Esnal D, Guelar A, Montero M, Guerri R, Sorli L, Lerma E, Villar J, Knobel H. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients Identified through a Multidisciplinary team. HIV clinical trials. 2018 Feb:19(1):1-7. doi: 10.1080/15284336.2017.1404690. Epub 2017 Nov 28
[PubMed PMID: 29179644]