Introduction
The sebaceous gland is integral to the structure and function of the skin, providing 90% of its surface lipids. While much of the focus relating to the sebaceous gland comes from its central role in acne vulgaris, several new functions have come to light that highlights this versatile cellular unit’s complex role in skin homeostasis. The sebaceous gland is special in at least two ways. Firstly, the product of this gland is synthesized via holocrine secretion, a unique method characterized by the purposeful self-destruction of its primary cellular unit, the sebocyte. Secondly, despite being of epithelial origin and possessing numerous hormone receptors, sebocytes engage in lipid synthesis and metabolism, a job normally reserved for adipocytes. Thus, the sebaceous gland can be considered both a hormonal target as well as an endocrine organ.[1][2][3] The production of sebum is the most important function of the sebaceous gland in humans. Unique to the sebaceous gland is the production of squalene and certain fatty acids. Sebum production is critical for maintaining skin homeostasis, lubrication, and physiological defense against environmental and infectious insults.[4][5] See Image. The Common Integument.
Cellular Level
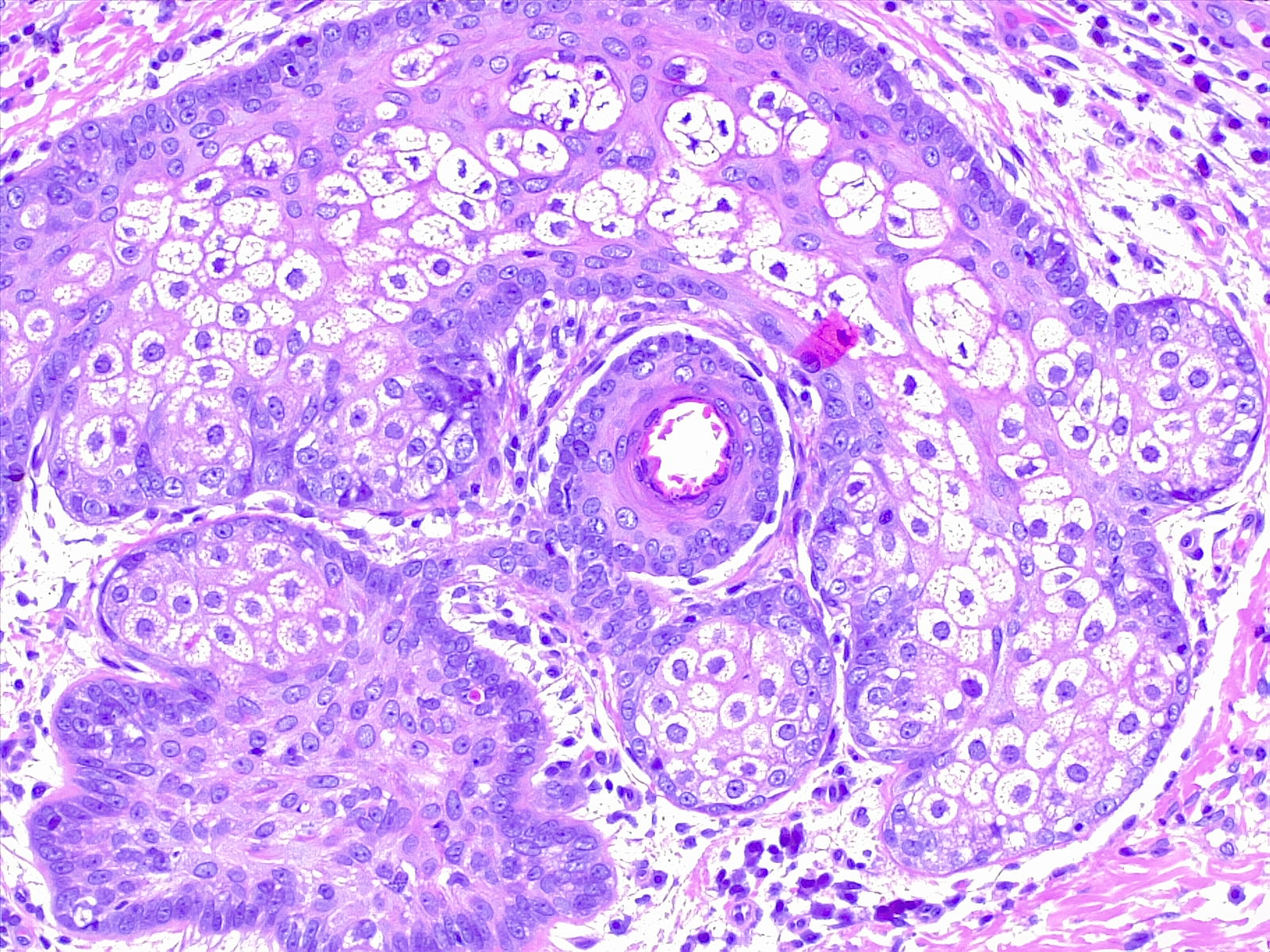
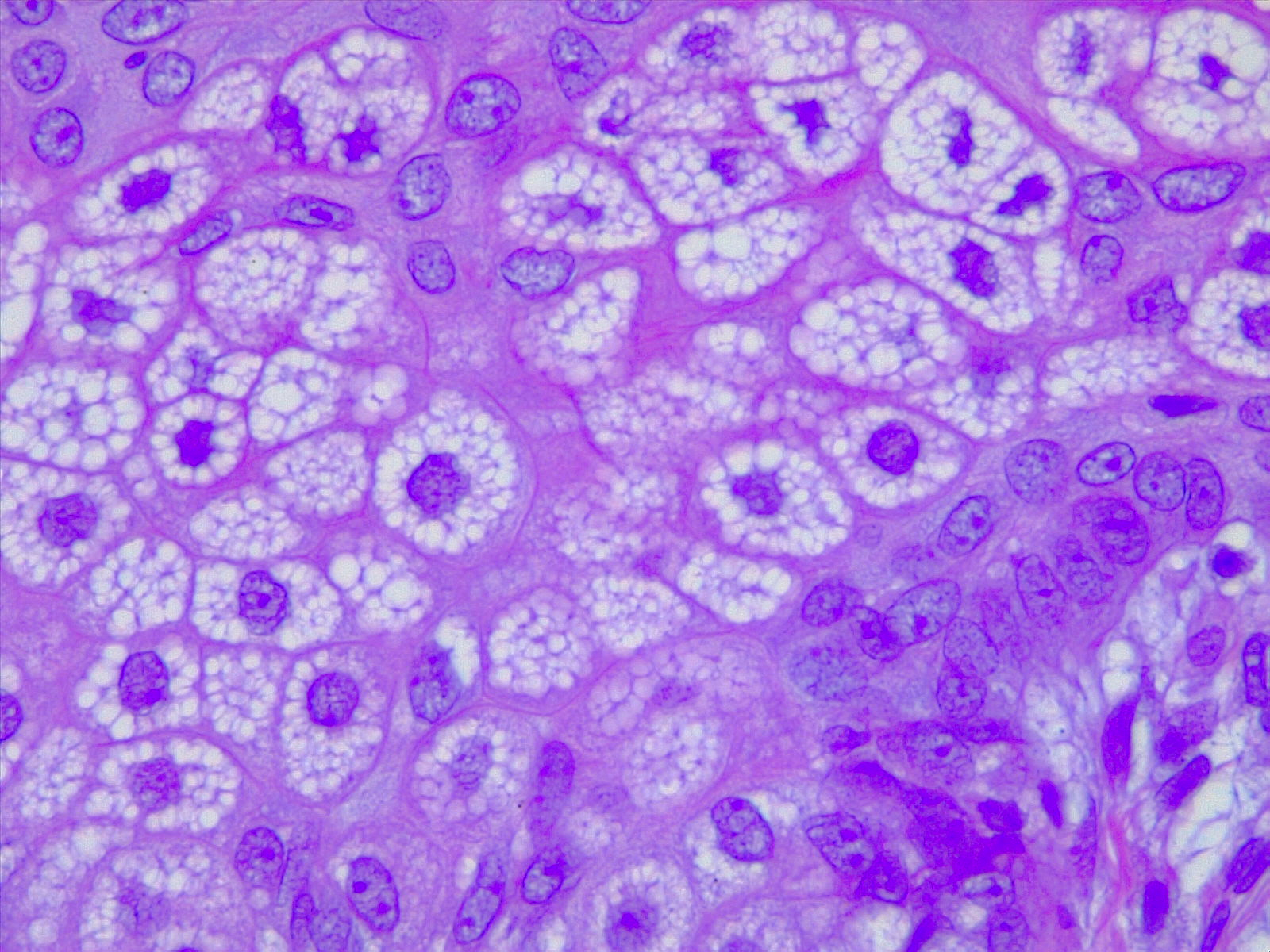
Sebaceous glands are grossly identifiable by their “foamy” appearance on microscopy (see Image. Sebaceous Glands, H/E 10x Magnification). The cells appear round and empty because their plentiful lipid contents are washed away during the staining process. The lipids may be visualized by using Nile red stain, a lipophilic stain. Relatively undifferentiated sebocytes are located along with the outer layers of the gland, gradually becoming more differentiated and filled with lipid products toward the center. At the center, mature sebocytes undergo apoptosis, degrade, and form the necrosis zone, eventually releasing their contents that combine to form sebum. See Image. Sebocytes, Sebaceous Gland Cells. This process of lipid synthesis and discharge takes about one week in total.
Development
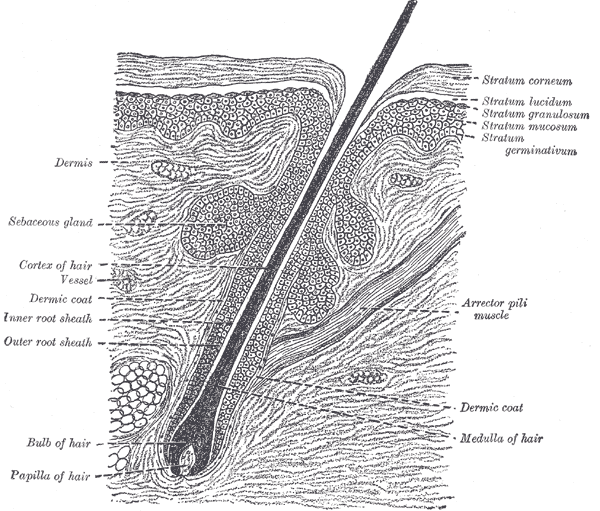
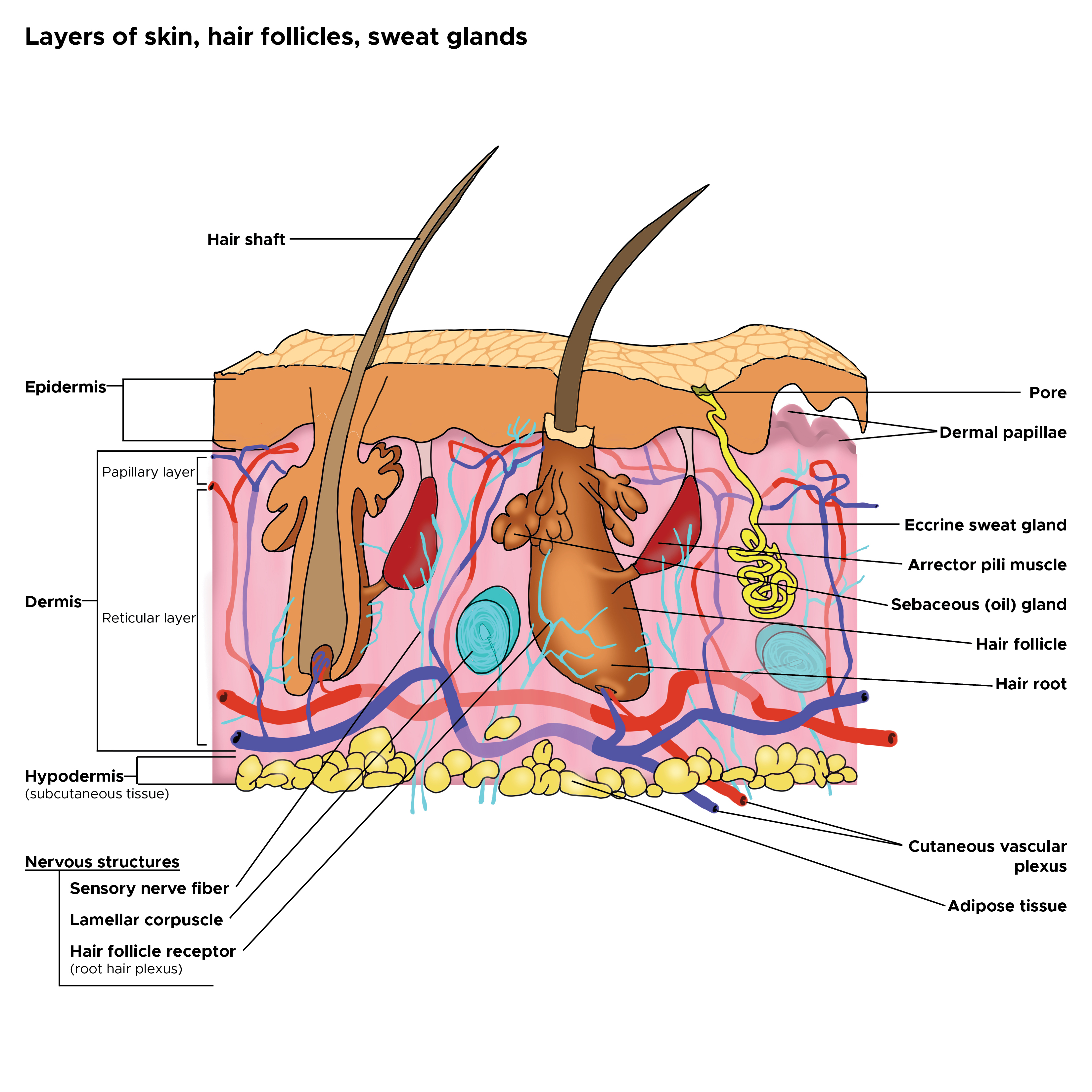
Sebaceous glands are located in the mid-dermis and almost always develop alongside a hair follicle, with an outlet emptying into the follicular canal. This association is known as the pilosebaceous unit. A small number of sebaceous glands known as Meibomian glands open directly onto the skin in the epithelium of the eyelid and help to enhance the lubricating properties of tears. See Image. Cross Section, Layers of the Skin.
The embryological development of the sebaceous gland lies in the process of ingrowth of ectodermal cells into the mesoderm. By weeks 13 to 15 of fetal life, the sebaceous gland can distinctly be visualized differentiating in a cephalocaudal sequence, in association with the hair follicle. At 17 weeks, lipid drops become visible at the center of the gland. The developing common excretory duct, which will serve as a focal point of attachment for the sebaceous gland acini, begins as a solid cord-like structure. The sebum-filled cells comprising the cord eventually rupture and form a channel that becomes the first pilosebaceous canal. The ductal opening of the sebaceous gland into the hair follicle is an important anatomical boundary; between this opening and the arrector pili muscle lies the bulge region of the hair follicle, a vital source of local stem cells. Interestingly, sebum is demonstrably the first glandular product made by the body. Sebaceous gland activity increases a few hours after birth and peaks during the first week of life, declining until the next peak during adrenarche (around nine years of age) until about 18 years of age. The approximate number of sebaceous glands generally remains constant throughout life, but their size tends to increase with age, especially during adolescence.[4]
Function
The sebaceous gland contributes the vast majority of skin surface lipids via its main product, sebum, which helps to seal in moisture and prevent desiccation of the skin. Because sebaceous glands empty into the hair canal, sebum mostly escapes onto the skin surface via a wicking action involving the hair shaft. In addition to cell debris and lipids, sebum also contains antimicrobial substances, free fatty acids, and matrix metalloproteinases. These elements, combined with the formation of a cutaneous lipid film, help protect the skin from external insults.[6][7]
The sebaceous gland is also an important site for androgen processing and modulation. All of the enzymes necessary for transforming cholesterol to steroids or adrenal precursors, such as dehydroepiandrosterone, are found in the skin. The sebaceous gland can also inactivate androgens via hydroxysteroid dehydrogenase, an enzyme present as early as 16 weeks of fetal life. The type-1 isoform of 5-alpha-reductase, which serves to convert testosterone into its most potent form, is also abundantly produced in the sebaceous glands, especially sebaceous glands found on the face and scalp.
The sebaceous gland is also importantly under hormonal control. Androgens regulate sebaceous gland function through binding to nuclear androgen receptors (AR). ARs are present in numerous skin components, with a particular predilection for the sebaceous gland, where androgens stimulate cell proliferation and lipogenesis.
Corticotropin-releasing hormone (CRH) stimulates local skin cell receptors via paracrine methods, leading to increased levels of proopiomelanocortin (POMC) and lowering IL-8 synthesis in sebocytes as well as inducing cortisol production. Together, these effects have a powerful anti-inflammatory effect that counteracts the normal stress-signal cascade and helps limit excessive tissue damage.
Clinical Significance
The most commonly discussed pathology relating to the sebaceous gland is acne vulgaris. Some estimate that up to 80% of people will experience acne at some point during their lifetime, and 20% of those will exhibit severe forms involving painful cysts, sinus tract formation, and permanent scarring. The pathogenesis of acne is complex, with the sebaceous gland playing a prominent role. During puberty, high levels of androgen production stimulate sebaceous glands to increased sebum production. This activity results in the formation of sebum plugs, which, combined with other factors, results in the characteristic open (blackheads) and closed (whiteheads) comedones of acne. Many individuals present with a form of acne at birth. Acne neonatorum, which presents within the first four weeks of life, occurs in up to 20% of newborns. Additionally, childhood acne is strongly correlated with the development of persistent acne later in life.[8][9]
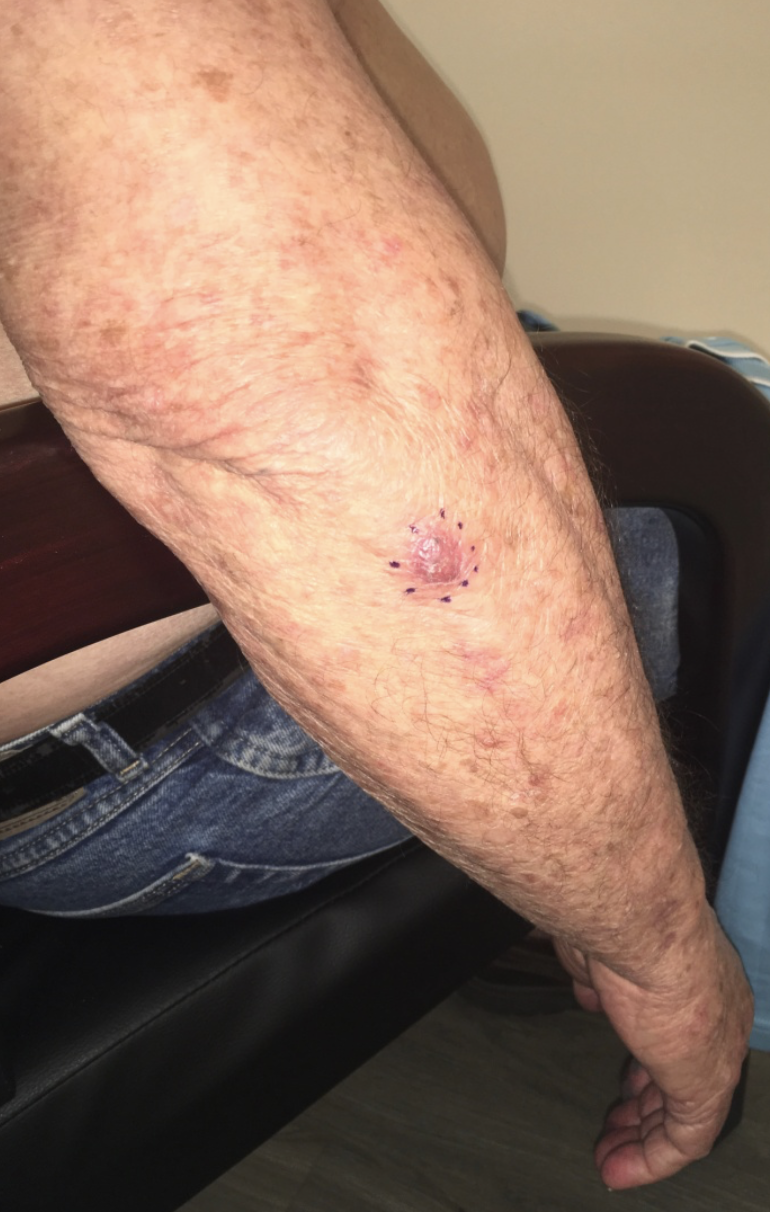
The sebaceous gland commonly takes on an enlarged appearance as patients age. This benign condition is known as sebaceous hyperplasia. While these small lesions are a purely cosmetic annoyance, they can appear similar to basal cell carcinoma and prompt unnecessary biopsy to the inexperienced practitioner. However, sebaceous glands can occasionally give rise to tumors, which are known as sebaceous adenomas (see Image. Sebaceous Adenoma). Muir-Torre syndrome, a rare, autosomal dominant condition, is characterized by sebaceous neoplasms as well as visceral malignancies. According to some studies, the high potential for metastasis and death in this syndrome (up to 25%) necessitates surgical removal of these lesions with a wide margin to reduce the risk of recurrence.[10][11]
Interestingly, the sebaceous gland also seems to be linked to androgenetic alopecia or pattern baldness, a genetic disorder involving the gradual diminishment in size, quality, and number of scalp hairs. Histologic examination of affected areas demonstrates a marked increase in the size of the associated sebaceous gland, suggesting that overgrowth of the gland and relative preservation of the follicular stem cells could be important factors in the etiology of this condition.