Introduction
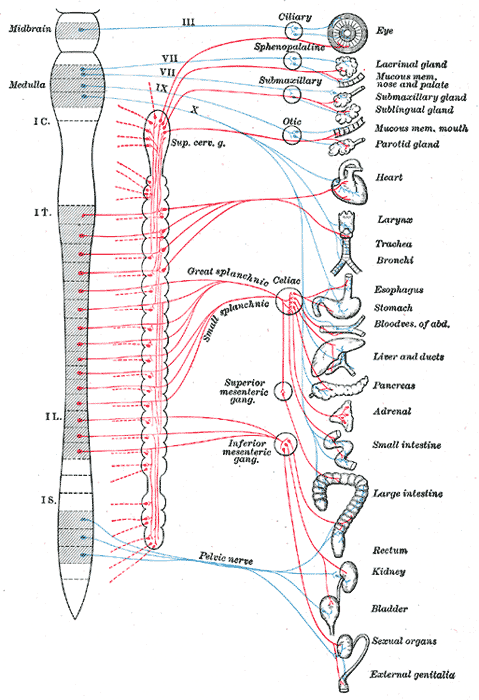
The parasympathetic nervous system (PNS) is one of the two functionally distinct and continuously active divisions of the autonomic nervous system (ANS). It is in opposition to the other, the sympathetic nervous system (SNS). The parasympathetic nervous system predominates in quiet “rest and digest” conditions while the sympathetic nervous system drives the “fight or flight” response in stressful situations (see Diagram. Diagram of Efferent Sympathetic (red) and Parasympathetic (blue) Nervous System). The main purpose of the PNS is to conserve energy to be used later and to regulate bodily functions like digestion and urination.[1]
Structure and Function
Both divisions of the ANS are comprised of a complex network of pathways responsible for maintaining the physiologic integrity of organs, tissues, and cells. They are composed of pre- and postganglionic neurons that act on effector organs.[2] The preganglionic neurons of the PNS come from brainstem nuclei and the sacral spinal cord (specifically S2-S4). The axons of preganglionic PNS neurons are much longer than those of the SNS and synapse with the postganglionic neurons in ganglia at or near the effector organs. The very short postganglionic axons then relay signals to the cells of the effector organs. Those preganglionic parasympathetic neurons that begin in the brainstem leave the central nervous system (CNS) through cranial nerves. Cranial nerves carrying parasympathetic functions include the oculomotor nerve (III) acting on the eyes, the facial nerve (VII) working on the lacrimal gland, the salivary glands, and the mucous membranes within the nasal cavity, the glossopharyngeal nerve (IX) acting on the parotid gland, and the vagus nerve (X) acting on the viscera of the abdomen and thorax. The vagus nerve is particularly influential within the PNS as it carries 75% of all parasympathetic fibers. The preganglionic fibers coming from the sacral cord join to form pelvic splanchnic nerves, which act on the pelvic cavity viscera.[1]
The PNS uses acetylcholine as its neurotransmitter for both pre- and postganglionic neurons activating muscarinic receptors. This differs from the SNS, which uses norepinephrine, which acts on adrenergic receptors, as the primary neurotransmitter for most postganglionic neurons. The primary exception is sweat glands stimulated by the SNS, which have cholinergic postganglionic neurons.[3] Muscarinic receptors are integral membrane proteins comprised of 5 subtypes (M1-M5) located on different effector organs. M1, M3, and M5 are coupled to Gq proteins, which signal through the IP3 pathway, while M2 and M4 receptors couple to Gi proteins, which signal through the cAMP pathway.[4] The type of receptor and its location are what determine the functions of the PNS.
Actions of the PNS are listed below:
- In the male reproductive tract, parasympathetic stimulation of M3 receptors causes smooth muscle relaxation in the helicine arteries of the penis, allowing blood to fill the corpora cavernosa and corpus spongiosum, causing an erection. The PNS also gives excitatory signals to the vas deferens, seminal vesicles, and prostate.[5]
- In the eye, parasympathetic stimulation of M3 receptors causes contraction of the sphincter muscle of the iris leading to constriction of the pupil (miosis). Additionally, it causes contraction of the ciliary muscle improving near vision.
- In the heart, parasympathetic stimulation of M2 receptors causes decreased heart rate and velocity of conduction through the AV node.
- In the vasculature, parasympathetic stimulation of M3 receptors leads to vasodilation.
- In the lungs, parasympathetic stimulation of M3 receptors leads to bronchoconstriction. It also increases bronchial secretions.
- In salivary glands, parasympathetic stimulation of M1 and M3 receptors leads to high volume secretion of potassium ions, water, and amylase.
- In the stomach and intestines, parasympathetic stimulation of M receptors leads to increased motility and relaxation of sphincters. Stimulation of M receptors also increases gastric secretions to aid in digestion.
- In the gallbladder, parasympathetic stimulation of M3 receptors stimulates contraction to release bile.
- In the pancreas, parasympathetic stimulation of M3 receptors leads to the release of digestive enzymes and insulin.
- In the kidneys and bladder, parasympathetic stimulation of M3 receptors stimulates peristalsis of ureters, contraction of the detrusor muscle, and relaxation of the internal urethral sphincter aiding in the flow and excretion of urine.
After being released from cholinergic neurons and acting on muscarinic receptors, acetylcholine is quickly inactivated or removed from the neuroeffector junction to allow new signals to come through. In cholinergic synapses, this action is primarily performed enzymatically by acetylcholinesterase. It hydrolyzes acetylcholine into choline and acetate in less than one millisecond, making it one of the fastest enzymes in the body.[1]
Embryology
Apart from a small contribution from neurogenic placodes to the ciliary ganglion and oculomotor nerves, the parasympathetic nervous system arises from neural crest cells.[6] The PNS specifically forms from cranial and sacral neural crest cells. The cranial neural crest cells become postganglionic neurons and glia within ganglia in the head, thorax, and abdomen, while the sacral neural crest cells postganglionic neurons in the pelvis.[7]
Physiologic Variants
Research has shown that respiratory variation of heart rate at the time of beta-blockade, which is a metric of cardiac PNS activity, decreases with age. Additionally, latency for the pupillary response to light, a metric of iris PNS activity, increases with age. Therefore, both studies have given a quantitative measure of the decrease in PNS activity with age.[8]
Surgical Considerations
Pelvic splanchnic nerve injury is a possible complication of surgery around the pelvic splanchnic nerves, such as rectal carcinoma resection. Damage to these nerves can cause autonomic dysregulation, such as urinary and sexual dysfunction.[9]
Vagotomy is a surgical procedure for patients with peptic ulcer disease refractive to treatment, which involves cutting fibers of the vagus nerve to reduce acidic secretions in the stomach. Though the procedure is no longer commonly used, there are still situations where it is an option. Due to disruption, a common complication is delayed gastric emptying. Furthermore, disruption of the vagus along the GI tract can cause gastroparesis.[10]
Vagus nerve stimulators are implantable in cases of intractable epilepsy and treatment-resistant depression.[11]
Clinical Significance
There are many ways that parasympathetic dysfunction can manifest, given the scope of organs on which it acts. Some examples of clinically significant conditions involving the PNS include sexual dysfunction, priapism, gastrointestinal issues, Horner syndrome, urinary retention, and cholinergic toxicity.
Sexual dysfunction due to physical trauma to splanchnic nerves, as mentioned above, or due to spinal cord lesions or neuropathy. Disruption of sexual function can also negatively impact mental health.[12] Conversely, if the sympathetic fibers become disrupted, the unopposed parasympathetic tone can result in priapism, that is, a painful erection lasting longer than four hours and is unrelated to sexual activity. Failure to treat priapism in time can result in irreversible corporal fibrosis.[13]
Dysmotility and decreased secretions can result from parasympathetic disruption within the gut. In turn, this can disrupt nutrient processing and absorption.[14]
Horner syndrome is a condition where parasympathetic output predominates due to disruption of sympathetic supply to the head. It can happen after a stroke or from systemic conditions like multiple sclerosis. Symptoms include ipsilateral ptosis, miosis, and anhidrosis.[15]
Urinary retention can occur as a result of spinal cord injury or compression or pelvic splanchnic nerve damage. Without the neurologic discernment of bladder volume, PNS stimulation of detrusor contraction and internal urethral sphincter relaxation may be incomplete or absent, leading to incomplete or absent voiding.[16][17]
Cholinergic drugs and organophosphates can cause cholinergic toxicity due to excessive cholinergic receptor stimulation. Symptoms of cholinergic toxicity can be remembered by the mnemonic, SLUDGE, which stands for salivation, lacrimation, urinary frequency, diarrhea/diaphoresis, gastrointestinal pain, and emesis.[18]